Question
Question: A cyclohexane ring is shown with two substituents. At the 1 position, there is a hydroxyl group (-OH...
A cyclohexane ring is shown with two substituents. At the 1 position, there is a hydroxyl group (-OH). At the 2 position, there is a carbon chain attached, which terminates in a hydroxyl group (-OH). This molecule is reacted with PCC in excess, yielding product A. Product A is then reacted with a diol (HO-CH2-CH2-OH) in the presence of H+ (1 equivalent), yielding product B. Product B is then reacted with CH3MgBr followed by H2O+, yielding product C. Product C is then reacted with NaBH4, yielding product D.
Identify A, B, C and D products.
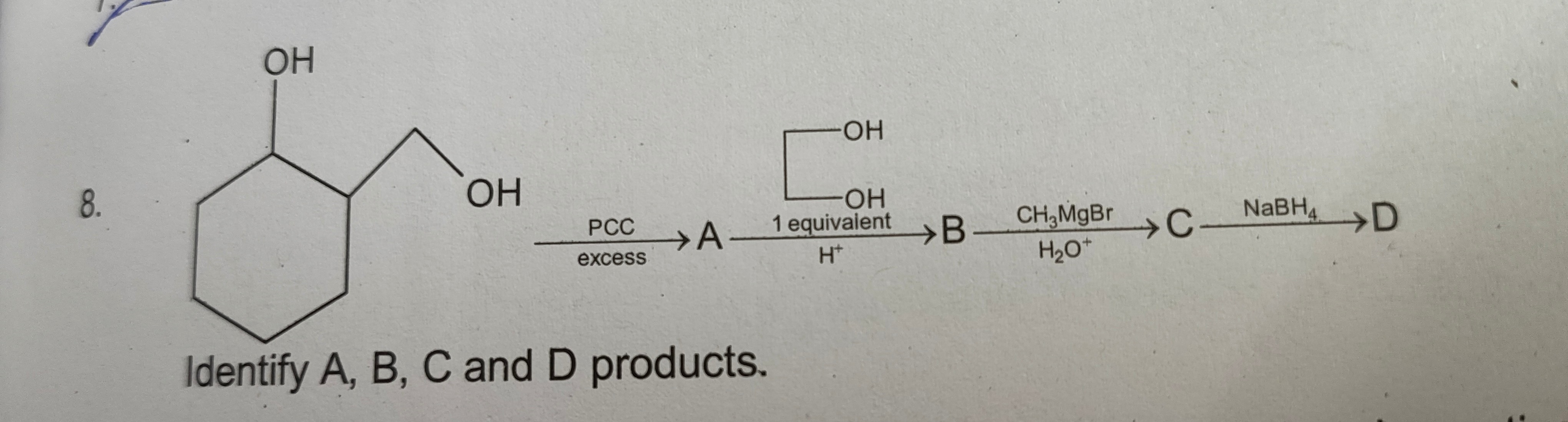
- A: 2‑(formylmethyl)cyclohexanone
- B: 2‑[(1,3‑dioxolane‑2‑yl)methyl]cyclohexanone
- C: 2‑[(1,3‑dioxolane‑2‑yl)methyl]‑1‑(hydroxy(CH₃))cyclohexane
- D: 2‑(2‑hydroxyethyl)‑1‑(hydroxy(CH₃))cyclohexane
Solution
Step‑1. (PCC oxidation)
- The secondary –OH (on the ring) is oxidized to give a cyclohexanone.
- The primary –OH (–CH₂CH₂OH) is oxidized only to the aldehyde stage (PCC being mild).
Thus, product A is:
A: 2‑(formylmethyl)cyclohexanone (That is, a cyclohexanone ring at C–1 with a –CH₂CHO group at C–2.)
Step‑2. (Acetal formation)
When A is treated with ethylene glycol (HO–CH₂CH₂–OH) in the presence of one equivalent of acid, the more reactive aldehyde is converted into its cyclic acetal (a 1,3‑dioxolane ring) while the ketone remains unaltered.
Thus, product B is:
B: 2‑[(1,3‑dioxolane‑2‑yl)methyl]cyclohexanone
Step‑3. (Grignard addition)
Treatment of B with CH₃MgBr followed by aqueous work‐up attacks the ketone (the cyclic acetal is inert to the nucleophile) giving after protonation the tertiary alcohol on the ring.
Thus, product C is:
C: 2‑[(1,3‑dioxolane‑2‑yl)methyl]‑1‑(hydroxy(CH₃))cyclohexane [Here the former ketone carbon now bears both an –OH and a –CH₃.]
Step‑4. (NaBH₄ reduction)
Finally, treatment of C with NaBH₄ under conditions that lead to acetal cleavage (usually the acetal is unmasked under the reaction work‐up) restores the carbonyl–derived group. However, in the presence of NaBH₄ the freshly “deprotected” aldehyde gets reduced to the primary alcohol.
Thus, product D is:
D: 2‑(2‑hydroxyethyl)‑1‑(hydroxy(CH₃))cyclohexane (that is, the –CH₂CHO group has been converted into –CH₂CH₂OH while the ring carbon remains as a tertiary alcohol from the Grignard addition).
