Question
Question: Correct order of bond angles in the given compounds is/are:...
Correct order of bond angles in the given compounds is/are:
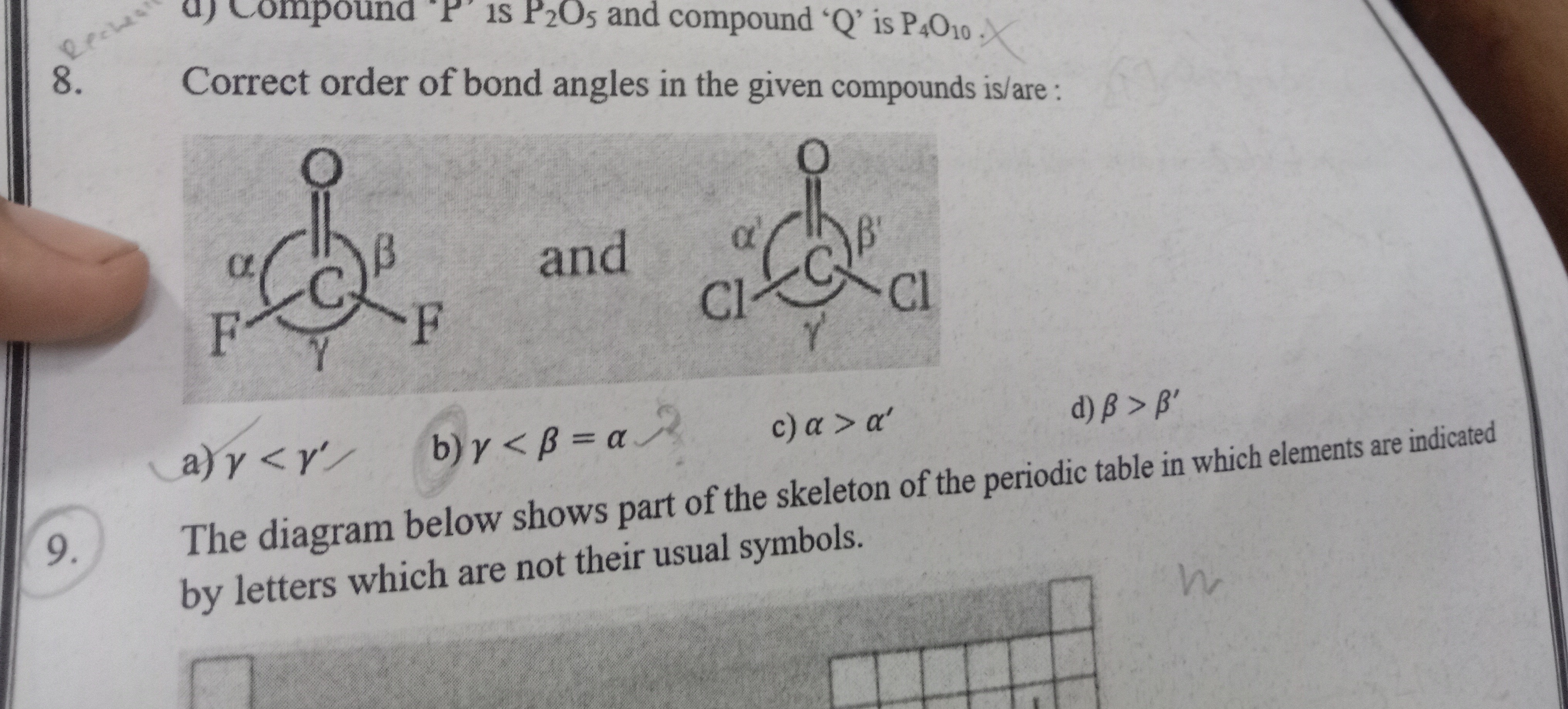
γ<γ′
γ<β=α
α>α′
β>β′
a) γ<γ′
Solution
In molecules like CF2O and CCl2O, the central carbon atom is sp2 hybridized, leading to a trigonal planar electron geometry. The C=O double bond exerts greater electron repulsion than the C−X single bonds (where X is F or Cl). This compression effect reduces the angles involving the C=O bond, making them less than 120∘, while the angle between the two C−X bonds is expanded, making it greater than 120∘. Thus, in both molecules, the angle between the two single bonds (γ and γ′) is larger than the angle between the C=O bond and a single bond (β and β′).
Comparing CF2O and CCl2O: Fluorine is more electronegative and smaller than chlorine. The greater electronegativity of fluorine leads to a more polarized C−F bond and potentially stronger electron withdrawal. This can result in a smaller bond angle between the C=O bond and the C−F bonds (β<β′), and also a smaller bond angle between the two C−F bonds compared to the two C−Cl bonds (γ<γ′). Therefore, the correct order is γ<γ′.
