Question
Question: Which of the following has the most negative heat of hydrogenation?...
Which of the following has the most negative heat of hydrogenation?
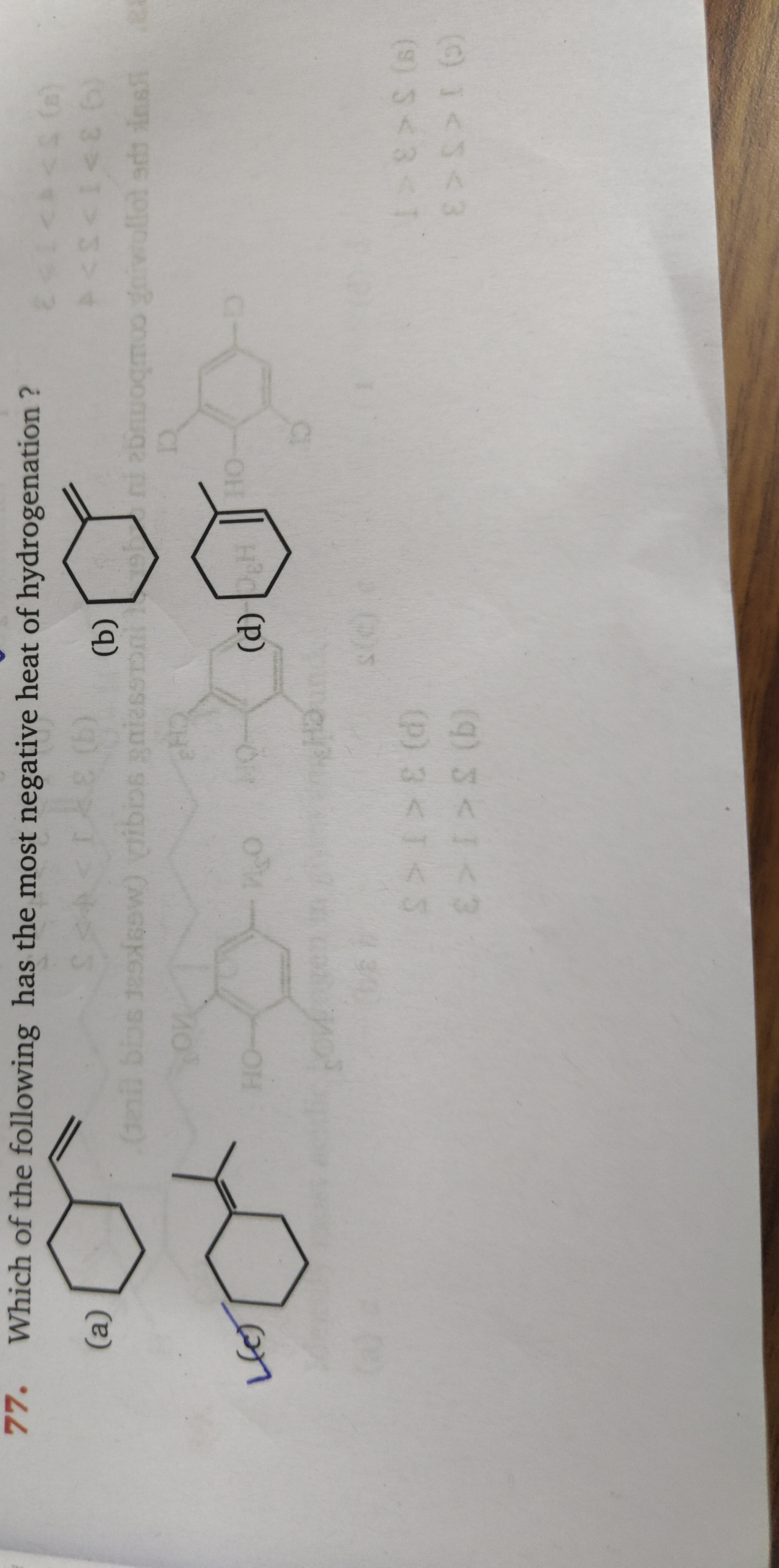
3 < 1 < 2
4 < 3 < 1
1 < 2 < 3
3 > 1 > 5
5 > 1 > 3
3 > 1 > 5
1 < 2 < 3
Solution
The heat of hydrogenation is the enthalpy change associated with the addition of hydrogen to an unsaturated compound to form a saturated compound. A more negative heat of hydrogenation indicates that more energy is released, meaning the unsaturated compound is less stable.
Let's analyze the structures, assuming they correspond to typical examples: Structure 1: A monosubstituted alkene (e.g., propene). Structure 2: A disubstituted alkene (e.g., cyclohexene). Structure 3: An aromatic compound (e.g., benzene). Structure 4: A tetrasubstituted alkene (e.g., tetramethylethylene). Structure 5: Not clearly defined in the options, but implied to be less stable than 1 and 3.
The stability of alkenes generally follows the order: tetrasubstituted > trisubstituted > disubstituted > monosubstituted > unsubstituted. More stable alkenes have less negative heats of hydrogenation. Therefore, for simple alkenes, the order of stability is 4>2>1. This implies the order of heat of hydrogenation from least negative to most negative is H4<H2<H1.
Now consider the aromatic compound, Structure 3 (benzene). Hydrogenation of an aromatic ring leads to the formation of a saturated cycloalkane and involves the loss of aromatic stabilization energy. This process is highly exothermic. The heat of hydrogenation of benzene is approximately -208 kJ/mol. For comparison, the heat of hydrogenation of cyclohexene (Structure 2) is approximately -118 kJ/mol, and for a monosubstituted alkene (Structure 1) it's around -120 kJ/mol. The heat of hydrogenation for a tetrasubstituted alkene (Structure 4) is around -113 kJ/mol.
Since the hydrogenation of an aromatic ring releases significantly more energy than the hydrogenation of simple alkenes, the aromatic compound (Structure 3) will have the most negative heat of hydrogenation.
Comparing the heats of hydrogenation: H3 (aromatic) ≈−208 kJ/mol H1 (monosubstituted alkene) ≈−120 kJ/mol H2 (disubstituted alkene) ≈−118 kJ/mol H4 (tetrasubstituted alkene) ≈−113 kJ/mol
The order of heat of hydrogenation from least negative to most negative is: H4<H2<H1<H3 (numerically: −113<−118<−120<−208). This is incorrect. The correct order of magnitude is: H4(−113)>H2(−118)>H1(−120)>H3(−208). So, the order from least negative to most negative is: H4<H2<H1<H3. This means the ranking should be 4 < 2 < 1 < 3.
The question asks "Which of the following has the most negative heat of hydrogenation?". This corresponds to the least stable compound. The aromatic system (3) is significantly less stable than simple alkenes due to the loss of aromaticity upon hydrogenation. Thus, it will have the most negative heat of hydrogenation.
Among the given options, option (c) is "1 < 2 < 3". If this represents the order of increasing heat of hydrogenation (from least negative to most negative), it implies H1<H2<H3. This correctly places H3 as the most negative. While the relative order of H1 and H2 can be debated or depend on specific substituents, the overwhelming difference in stability and heat of hydrogenation for the aromatic ring makes it the clear answer for the most negative value. Therefore, option (c) is the best fit, indicating that compound 3 has the most negative heat of hydrogenation compared to compounds 1 and 2.
