Question
Question: Which of the following, is the product of the reaction between $AlCl_3$ and $CH_3OCH_3$?...
Which of the following, is the product of the reaction between AlCl3 and CH3OCH3?
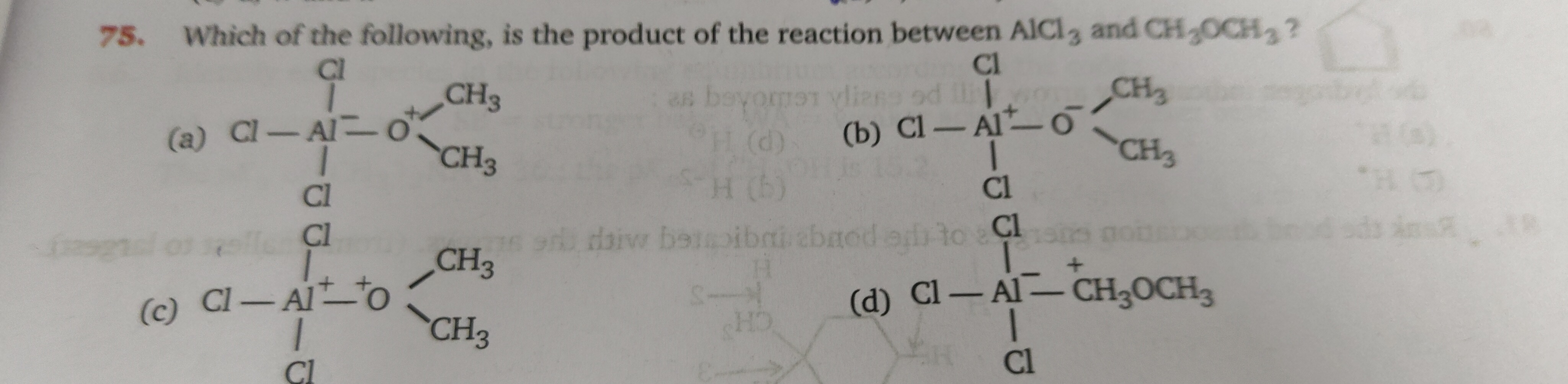
A
Cl - Cl∣ClAl− - O+CH3CH3
B
Cl - Cl∣ClAl+ - O−CH3CH3
C
Cl - Cl∣ClAl+ - O+CH3CH3
D
Cl - Cl∣ClAl− - CH3OCH3
Answer
Cl - Cl∣ClAl− - O+CH3CH3
Explanation
Solution
AlCl3 acts as a Lewis acid due to the incomplete octet on the aluminum atom. CH3OCH3 (dimethyl ether) acts as a Lewis base, with the oxygen atom donating a lone pair of electrons. The reaction forms a coordinate covalent bond between the oxygen atom of dimethyl ether and the aluminum atom of AlCl3. This results in a formal positive charge on the oxygen atom and a formal negative charge on the aluminum atom. The structure of the adduct formed is:
Cl
|
Cl-Al⁻ - O⁺
| /
Cl CH3 CH3
