Question
Question: $Mg_2C_3 + H_2O \longrightarrow A \xrightarrow{Na} B \xrightarrow{CH_3Br} C;$ The incorrect statem...
Mg2C3+H2O⟶ANaBCH3BrC;
The incorrect statement for C is
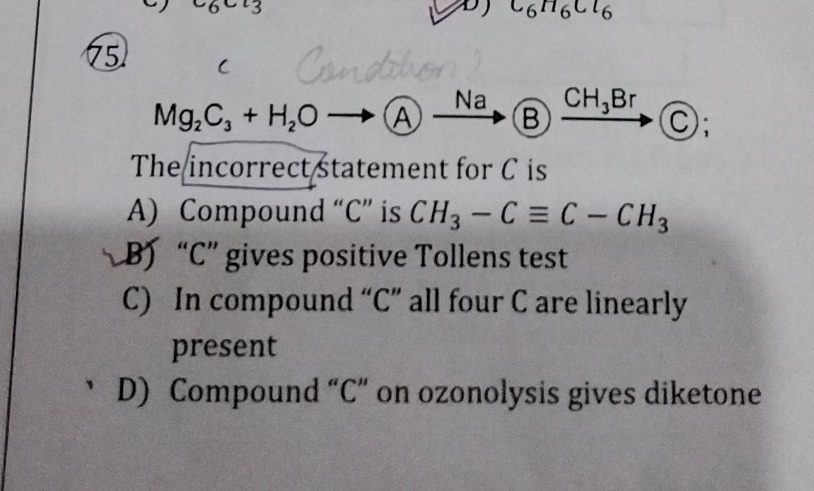
Compound "C" is CH3−C≡C−CH3
"C" gives positive Tollens test
In compound "C" all four C are linearly present
Compound "C" on ozonolysis gives diketone
"C" gives positive Tollens test
Solution
The problem asks us to identify the incorrect statement about compound 'C' formed in the given reaction sequence.
Step-by-step derivation of compounds A, B, and C:
-
Mg2C3+H2O⟶A
Magnesium carbide (Mg2C3) is an ionic carbide that contains the propadienylide ion (C34−). Upon hydrolysis, it produces propyne (methylacetylene).
Mg2C3+4H2O⟶2Mg(OH)2+CH3−C≡CH
So, compound A is propyne (CH3−C≡CH).
-
ANaB
Propyne (A) is a terminal alkyne, meaning it has an acidic hydrogen atom attached to the triple bond. It reacts with active metals like sodium to form a sodium acetylide (alkynide).
CH3−C≡CH+Na⟶CH3−C≡C−Na++21H2
So, compound B is sodium propynide (CH3−C≡C−Na+).
-
BCH3BrC
Sodium propynide (B) is a strong nucleophile and undergoes an SN2 reaction with methyl bromide (CH3Br), which is an alkyl halide. This is an alkyne alkylation reaction.
CH3−C≡C−Na++CH3Br⟶CH3−C≡C−CH3+NaBr
So, compound C is but-2-yne (CH3−C≡C−CH3).
Now, let's evaluate the given statements about compound C (but-2-yne):
A) Compound "C" is CH3−C≡C−CH3
This statement is correct, as derived above.
B) "C" gives positive Tollens test
Tollens' reagent (ammoniacal silver nitrate) is used to detect terminal alkynes (those with an acidic hydrogen, i.e., −C≡CH group). But-2-yne (CH3−C≡C−CH3) is an internal alkyne; it does not have an acidic hydrogen atom directly attached to the triple bond. Therefore, it will not give a positive Tollens test.
This statement is incorrect.
C) In compound "C" all four C are linearly present
In but-2-yne, the two carbon atoms involved in the triple bond are sp-hybridized. The bond angle around an sp-hybridized carbon atom is 180 degrees. This means the carbon atoms directly attached to the triple bond carbons also lie in a straight line with the triple bond. Thus, the C−C≡C−C backbone of but-2-yne is linear.
This statement is correct.
D) Compound "C" on ozonolysis gives diketone
Ozonolysis of internal alkynes generally leads to the oxidative cleavage of the triple bond, yielding carboxylic acids. For but-2-yne, the products are two molecules of acetic acid:
CH3−C≡C−CH3O3,H2O2CH3COOH
Formation of a diketone (CH3−CO−CO−CH3) is not the typical or final product of standard ozonolysis of internal alkynes. While α-diketones might be transient intermediates or formed under very specific non-standard conditions (e.g., low temperature, reductive workup with specific reagents), the general statement "gives diketone" is considered incorrect in the context of standard ozonolysis. Therefore, this statement is also incorrect under typical conditions.
Conclusion:
Both statements B and D appear to be incorrect based on standard chemical knowledge. However, in multiple-choice questions where only one option is expected to be incorrect, we look for the most definitively incorrect statement.
Statement B is unequivocally incorrect: internal alkynes fundamentally lack the acidic proton required for a positive Tollens test.
Statement D is incorrect under standard ozonolysis conditions, but some specialized conditions or consideration of intermediates might lead to diketones. However, for general reactions, carboxylic acids are the expected products.
Given the options, statement B is a more fundamental and universally accepted incorrect statement regarding the properties of but-2-yne.
