Question
Question: $n$ moles of He gas is placed in a vessel of volume $V$ L at $T$ K. If $V_1$ is free volume of He, t...
n moles of He gas is placed in a vessel of volume V L at T K. If V1 is free volume of He, then the diameter of He atom is
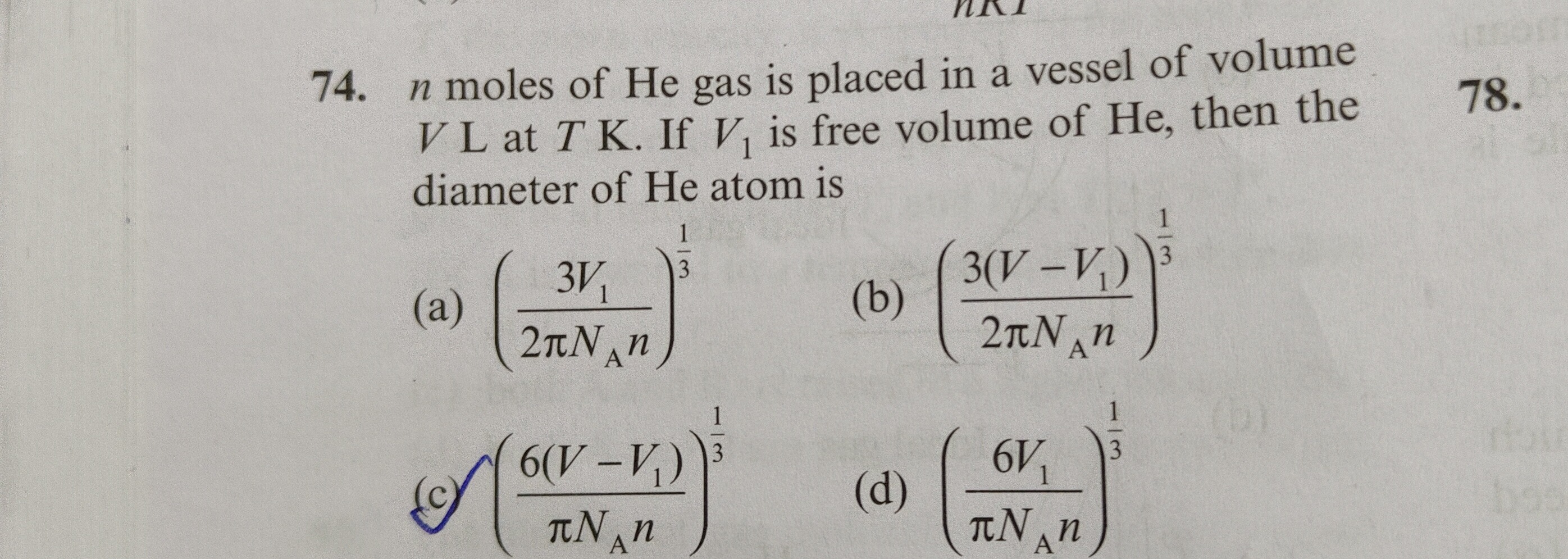
A
(a) (2πNAn3V1)31
B
(b) (2πNAn3(V−V1))31
C
(c) (πNAn6(V−V1))31
D
(d) (πNAn6V1)31
Answer
(c) (πNAn6(V−V1))31
Explanation
Solution
The volume occupied by n moles of He atoms is V−V1. This volume equals nNAvatom, where vatom is the volume of a single atom. Thus, vatom=nNAV−V1. Assuming the atom is a sphere with radius r, vatom=34πr3. Equating these, 34πr3=nNAV−V1. Solving for the diameter d=2r, we get d=(πnNA6(V−V1))1/3.
