Question
Question: In which of the following compound size of cation to anion ratio is minimum:...
In which of the following compound size of cation to anion ratio is minimum:
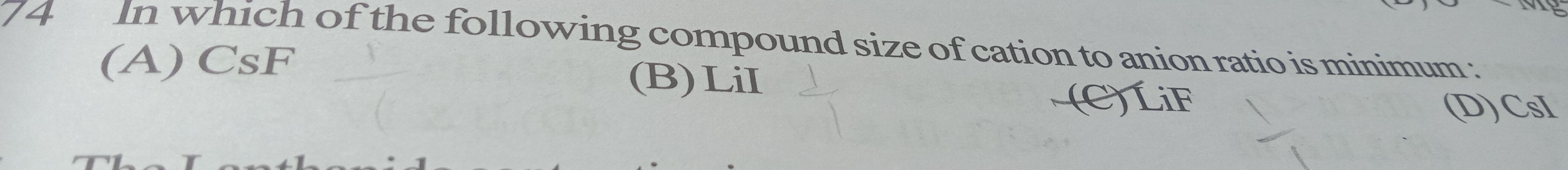
A
CsF
B
LiI
C
LiF
D
CsI
Answer
LiI
Explanation
Solution
To minimize the cation to anion size ratio (rcation/ranion), the cation size must be the smallest and the anion size must be the largest. Among the given options, Li+ is the smallest cation and I− is the largest anion. Therefore, LiI has the minimum cation to anion size ratio.
