Question
Question: $$CH_3-C \equiv C-CH_3 \xrightarrow{Na/liq. NH_3} (A);$$ $$CH_3-C \equiv C-CH_3 \xrightarrow[\left(C...
CH3−C≡C−CH3Na/liq.NH3(A); CH3−C≡C−CH3Pd/CaCO3(CH3COO)2Pb(B); The minimum heat of hydrogenation is in
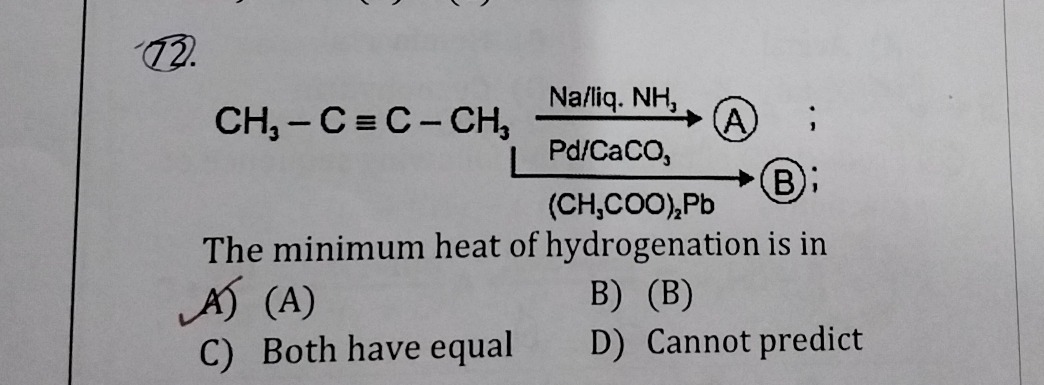
A
(A)
B
(B)
C
Both have equal
D
Cannot predict
Answer
A
Explanation
Solution
(A) is trans-but-2-ene, and (B) is cis-but-2-ene.Trans-but-2-ene is more stable than cis-but-2-ene. More stable isomers have lower energy and thus release less heat upon hydrogenation to the same product (butane). Therefore, (A) will have the minimum heat of hydrogenation.
