Question
Question: Consider the following reaction, Which of the following statements via $S_N2$ is correct?...
Consider the following reaction,
Which of the following statements via SN2 is correct?
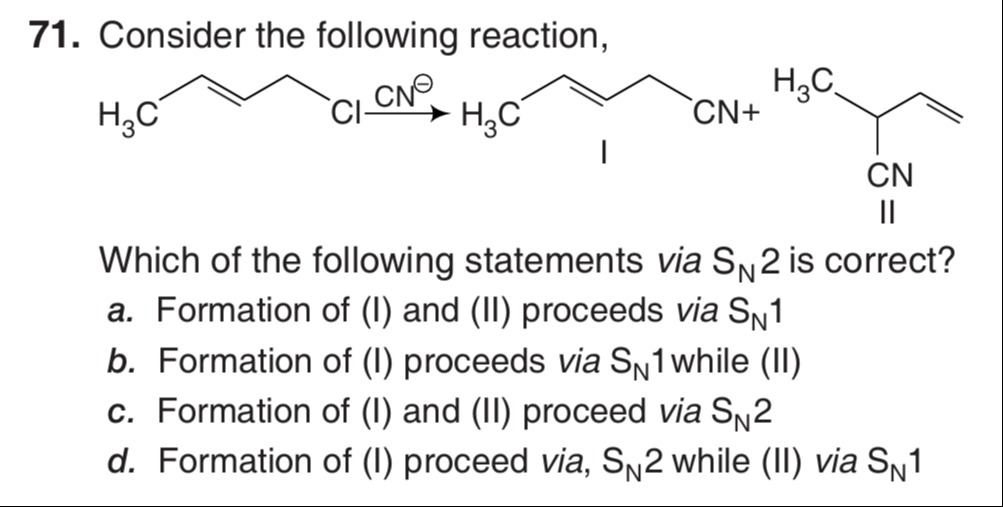
Formation of (I) and (II) proceeds via SN1
Formation of (I) proceeds via SN1 while (II)
Formation of (I) and (II) proceed via SN2
Formation of (I) proceed via, SN2 while (II) via SN1
d. Formation of (I) proceed via, SN2 while (II) via SN1
Solution
The reaction involves a primary allylic halide reacting with a strong nucleophile (CN⁻). The substrate is 1-chloro-3-pentene: CH₃CH=CHCH₂Cl. Product (I) is 1-cyano-3-pentene: CH₃CH=CHCH₂CN. This is the direct substitution product. Product (II) is 3-cyano-1-pentene: CH₃CH(CN)CH=CH₂. This is the rearranged substitution product.
Let's consider the possible mechanisms:
-
SN2: The nucleophile attacks the carbon bearing the leaving group from the backside. This leads to the formation of product (I).
CH₃CH=CHCH₂Cl + CN⁻ → CH₃CH=CHCH₂CN + Cl⁻
-
SN1: The leaving group departs to form a carbocation. Since the substrate is allylic, a resonance-stabilized carbocation is formed.
CH₃CH=CHCH₂Cl → CH₃CH=CHCH₂⁺ + Cl⁻ ↔ CH₃CH⁺CH=CH₂
The nucleophile can attack either carbon with a partial positive charge.
Attack at the primary carbon (original position of Cl): CH₃CH=CHCH₂⁺ + CN⁻ → CH₃CH=CHCH₂CN (Product I)
Attack at the secondary carbon (rearranged position): CH₃CH⁺CH=CH₂ + CN⁻ → CH₃CH(CN)CH=CH₂ (Product II)
-
SN2′: The nucleophile attacks the carbon two positions away from the leaving group (γ-carbon), the double bond shifts, and the leaving group departs from the original carbon (α-carbon).
CH₃CH=CHCH₂Cl + CN⁻ → [Transition state] → CH₃CH(CN)CH=CH₂ + Cl⁻ (Product II)
With a primary allylic halide and a strong nucleophile, SN2 and SN2′ are significant pathways. SN1 is also possible due to the resonance-stabilized allylic carbocation.
Considering the options, option d provides a reasonable description of possible pathways for the formation of the products. Product (I) is the direct substitution product, which is typically formed by SN2 with primary halides and strong nucleophiles. Product (II) is the rearranged product, which can be formed via SN1 (attack on the resonance-stabilized carbocation) or SN2′. Since SN1 is listed as a possibility for (II), and SN2 for (I), option d is the most likely correct answer among the given choices, assuming these are significant pathways.
