Question
Question: The correct information(s) about the n-factor of given species in the reaction: $KMnO_4 + H_2SO_4 + ...
The correct information(s) about the n-factor of given species in the reaction: KMnO4+H2SO4+HCl→K2SO4+MnSO4+Cl2+H2O is/are -
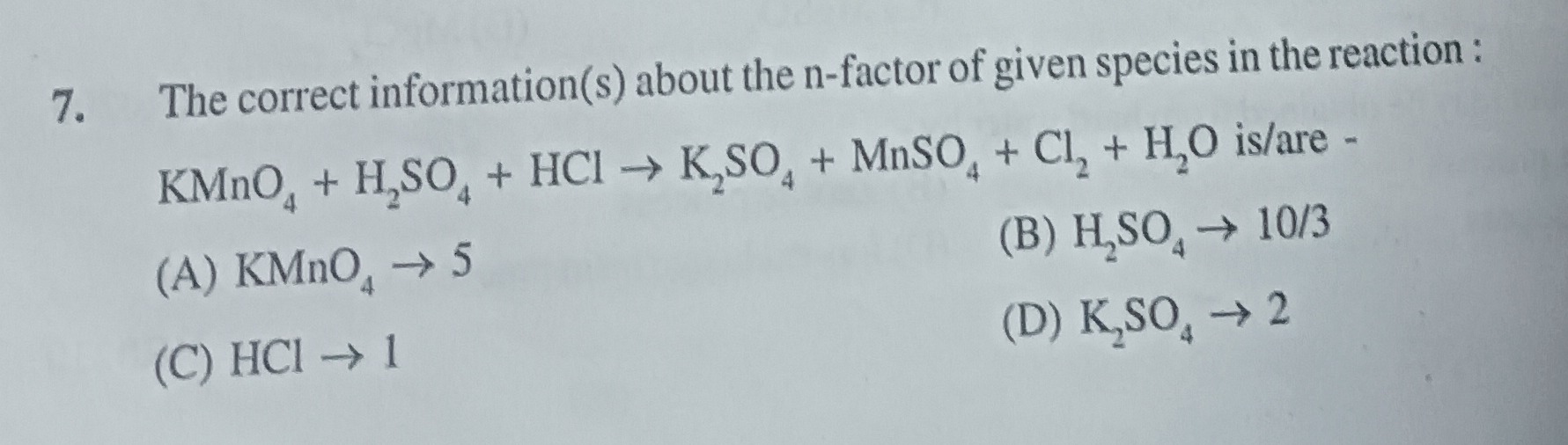
KMnO4→5
H2SO4→10/3
HCl→1
K2SO4→2
A, C
Solution
To determine the n-factor (also known as the equivalence factor) for each species in a redox reaction, we need to identify the change in the oxidation state of the elements involved in the electron transfer. The n-factor is defined as the number of electrons gained or lost per mole of the substance.
The given unbalanced reaction is: KMnO4+H2SO4+HCl→K2SO4+MnSO4+Cl2+H2O
First, let's determine the oxidation states of the relevant elements:
-
Manganese (Mn):
- In KMnO4: K is +1, O is -2. Let Mn be x. 1+x+4(−2)=0⇒x=+7.
- In MnSO4: SO4 is a sulfate ion with a -2 charge, so S is +6 and O is -2. Let Mn be y. y+(−2)=0⇒y=+2.
- Change in oxidation state for Mn: From +7 to +2. This is a gain of 5 electrons.
- n-factor for KMnO4 = 5. (Since 1 mole of KMnO4 contains 1 mole of Mn, and Mn gains 5 electrons).
-
Chlorine (Cl):
- In HCl: H is +1. Let Cl be z. 1+z=0⇒z=−1.
- In Cl2: Elemental chlorine has an oxidation state of 0.
- Change in oxidation state for Cl: From -1 to 0. This is a loss of 1 electron per Cl atom.
- n-factor for HCl = 1. (Since 1 mole of HCl contains 1 mole of Cl, and Cl loses 1 electron).
-
Sulfur (S):
- In H2SO4: H is +1, O is -2. Let S be w. 2(+1)+w+4(−2)=0⇒w=+6.
- In K2SO4 and MnSO4: S remains in the +6 oxidation state.
- Since there is no change in the oxidation state of Sulfur, H2SO4 acts as an acid (providing H+ ions) and not as a redox species.
- n-factor for H2SO4 in this redox reaction = 0.
-
Potassium Sulfate (K2SO4):
- This is a product of the reaction. K is +1, S is +6, O is -2. None of these elements change their oxidation states during the reaction. K2SO4 is a salt formed by spectator ions (K+ and SO42−).
- n-factor for K2SO4 in this redox reaction = 0.
Now, let's evaluate the given options:
(A) KMnO4→5: This matches our calculation. Correct.
(B) H2SO4→10/3: Our calculation shows n-factor = 0. So, this is incorrect.
(C) HCl→1: This matches our calculation. Correct.
(D) K2SO4→2: Our calculation shows n-factor = 0. So, this is incorrect.
Thus, the correct information(s) are (A) and (C).
The balanced chemical equation for completeness is: 2KMnO4+10HCl+3H2SO4→2MnSO4+5Cl2+K2SO4+8H2O
