Question
Question: Identify the reactants (X) and (Y) for the following reaction, respectively. (X) + (Y) $\xrightarro...
Identify the reactants (X) and (Y) for the following reaction, respectively.
(X) + (Y) NaNH2 4- Decyne Alkyl halide Alkyne
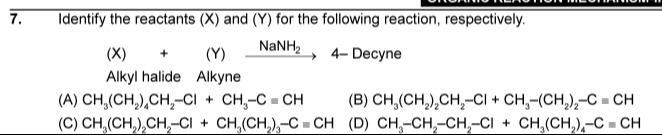
CH3(CH2)4CH2-CI + CH3-C ≡ CH
CH3(CH2)2CH2-CI + CH3-(CH2)2-C ≡ CH
CH3(CH2)2CH2-CI + CH3(CH2)3-C≡CH
CH3-CH2-CH2-CI + CH3(CH2)4-C≡CH
D
Solution
The reaction shown is the synthesis of an internal alkyne by the alkylation of a terminal alkyne using a strong base like NaNH2. The general reaction is:
R−C≡CH+R′−XNaNH2R−C≡C−R′+NaX+NH3
Here, (Y) is the terminal alkyne (R−C≡CH) and (X) is the alkyl halide (R′−X). NaNH2 deprotonates the terminal alkyne to form the acetylide anion (R−C≡C−), which then acts as a nucleophile in an SN2 reaction with the alkyl halide R′−X to form the new carbon-carbon bond and produce the internal alkyne R−C≡C−R′.
The product is given as 4-Decyne, which has the structure CH3−CH2−CH2−C≡C−CH2−CH2−CH2−CH2−CH3.
Option (D) yields 4-Decyne: (X) = CH3−CH2−CH2−Cl (1-Propyl chloride) (Y) = CH3(CH2)4−C≡CH (1-Heptyne)
