Question
Question: Bond order of S-O bond in $SO_3^2$ is [NCERT Pg. 109] ...
Bond order of S-O bond in SO32 is [NCERT Pg. 109]
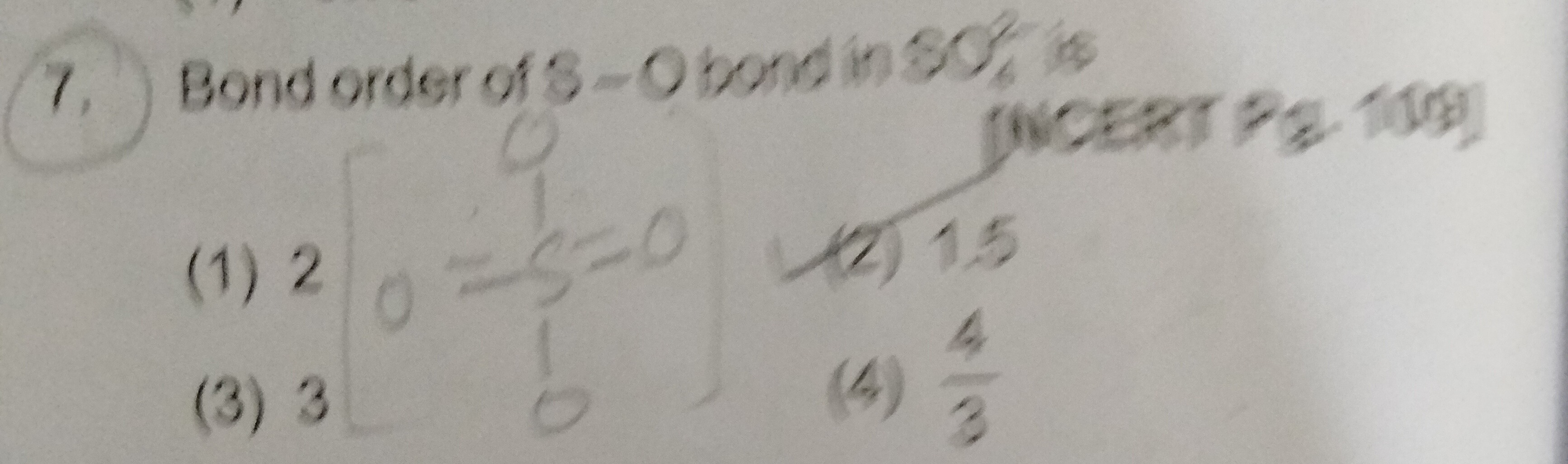
2
1.5
3
34
34
Solution
To determine the bond order of the S-O bond in SO32− (sulfite ion), we follow these steps:
-
Calculate the total number of valence electrons:
- Sulfur (S) is in Group 16, so it has 6 valence electrons.
- Oxygen (O) is in Group 16, so each has 6 valence electrons. There are 3 oxygen atoms.
- The charge of the ion is -2, so we add 2 electrons. Total valence electrons = 6 (for S) + 3 × 6 (for 3 O) + 2 (for charge) = 6 + 18 + 2 = 26 electrons.
-
Draw the Lewis structure:
- Place sulfur as the central atom, as it is less electronegative than oxygen.
- Connect the central sulfur atom to the three oxygen atoms with single bonds. This uses 3 × 2 = 6 electrons. Remaining electrons = 26 - 6 = 20 electrons.
- Distribute the remaining electrons to the terminal oxygen atoms to complete their octets. Each oxygen needs 6 more electrons (3 lone pairs). 3 O atoms × 6 electrons/O = 18 electrons used. Remaining electrons = 20 - 18 = 2 electrons.
- Place the last 2 electrons on the central sulfur atom as a lone pair.
The initial Lewis structure (with all single bonds):
:O: | :O-S-O: | :O:(Each oxygen has 3 lone pairs, sulfur has 1 lone pair)
-
Calculate formal charges for the initial structure:
- Formal charge on Oxygen = 6 - (6 lone pair electrons + 1/2 × 2 bonding electrons) = 6 - (6 + 1) = -1. (All three oxygens have -1 formal charge).
- Formal charge on Sulfur = 6 - (2 lone pair electrons + 1/2 × 6 bonding electrons) = 6 - (2 + 3) = +1. The sum of formal charges = 3(-1) + 1(+1) = -2, which matches the ion's charge.
-
Consider resonance structures to minimize formal charges (if possible) and account for delocalization:
Sulfur is in Period 3 and can expand its octet. To minimize formal charges, a lone pair from one of the oxygen atoms can be moved to form a double bond with sulfur. This will make the formal charge on that oxygen 0 and on sulfur 0.
Let's form a double bond with one of the oxygen atoms:
:O: || :O-S-O: | :O:-(The double-bonded oxygen has 2 lone pairs, formal charge = 0. The two single-bonded oxygens each have 3 lone pairs, formal charge = -1. Sulfur has 1 lone pair, formal charge = 0, and 10 electrons around it, expanding its octet).
Since there are three equivalent oxygen atoms, the double bond can be formed with any of the three oxygen atoms. This leads to three equivalent resonance structures:
Resonance Structure 1:
:O: || :O-S-O: | :O:-Resonance Structure 2:
:O:- | :O=S-O: | :O:-Resonance Structure 3:
:O:- | :O-S=O: | :O:-These three structures contribute equally to the resonance hybrid.
-
Calculate the bond order:
The bond order is calculated as the total number of bonds (sigma + pi) between the central atom and a peripheral atom, divided by the number of equivalent bond positions.
In any one of the resonance structures, there is:
- One S=O double bond (contributes 2 to the bond count).
- Two S-O single bonds (each contributes 1 to the bond count). Total number of bonds around sulfur in one canonical structure = 2 (from double bond) + 1 (from first single bond) + 1 (from second single bond) = 4 bonds.
These 4 "bond units" are delocalized over 3 equivalent S-O positions.
Bond Order = (Total number of bonds of a given type in all resonance structures) / (Number of resonance structures) Alternatively, for a specific bond: Bond Order = (Sum of bond orders for a particular S-O bond in all resonance structures) / (Number of resonance structures)
Let's consider one specific S-O bond (e.g., the top one):
- In Structure 1, it is a double bond (bond order = 2).
- In Structure 2, it is a single bond (bond order = 1).
- In Structure 3, it is a single bond (bond order = 1).
Average Bond Order = (2 + 1 + 1) / 3 = 4 / 3.
The bond order of the S-O bond in SO32− is 34.
