Question
Question: $\begin{array}{c} \text{CH}_3 \\ \text{I} \\ \text{C} \\ \text{III} \\ \text{CH} \end{array} \xright...
CH3ICIIICHKMnO4H2SO4ANaOHCaOB; Compound B is
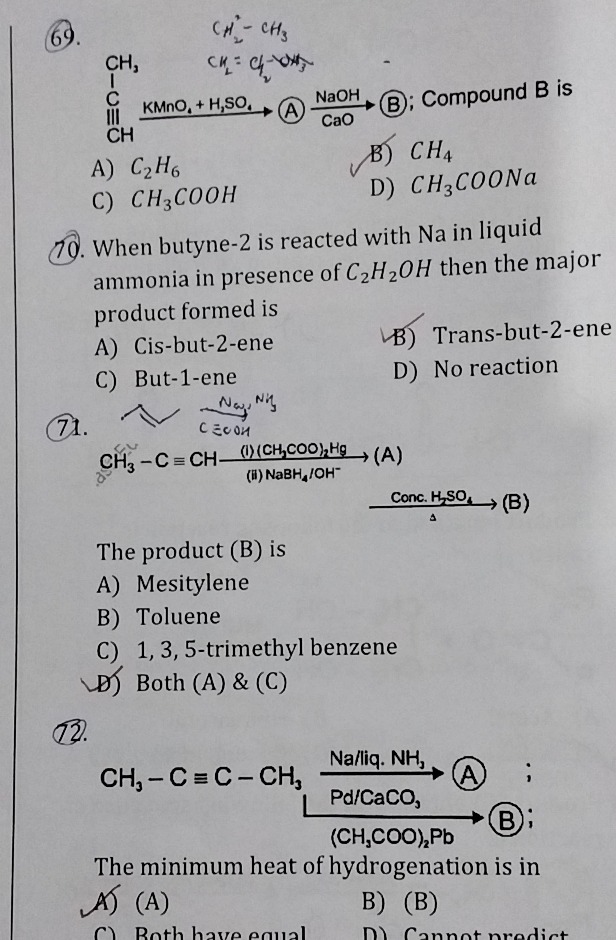
C2H6
CH4
CH3COOH
CH3COONa
CH4
Solution
The reaction proceeds in two steps:
-
Oxidation of Propyne (CH₃-C≡CH) with KMnO₄/H₂SO₄: Strong oxidizing agents like hot acidic KMnO₄ cause oxidative cleavage of alkynes. For terminal alkynes, the triple bond breaks, and the terminal
≡CHgroup is oxidized toCO₂. The other part of the alkyne (CH₃-C≡) is oxidized to a carboxylic acid (CH₃COOH).CH₃-C≡CH + [O] --(KMnO₄, H₂SO₄)--> CH₃COOH + CO₂So, compound A is Acetic Acid (
CH₃COOH). -
Decarboxylation of Acetic Acid (A) with NaOH/CaO: This is a soda lime decarboxylation reaction. Carboxylic acids (or their sodium salts, which would be formed in situ with NaOH) undergo decarboxylation when heated with soda lime (a mixture of NaOH and CaO), losing a carbon atom as
CO₂(which formsNa₂CO₃).CH₃COOH + NaOH --(CaO, Δ)--> CH₄ + Na₂CO₃(More accurately,
CH₃COONa + NaOH --(CaO, Δ)--> CH₄ + Na₂CO₃)So, compound B is Methane (
CH₄).
Compound B is CH₄.
