Question
Question: 672 ml of ozonized oxygen $(0_3+0_2)$ were found to weight 1g Calculate volume of oronized oxygen....
672 ml of ozonized oxygen (03+02) were found to weight 1g Calculate volume of oronized oxygen.
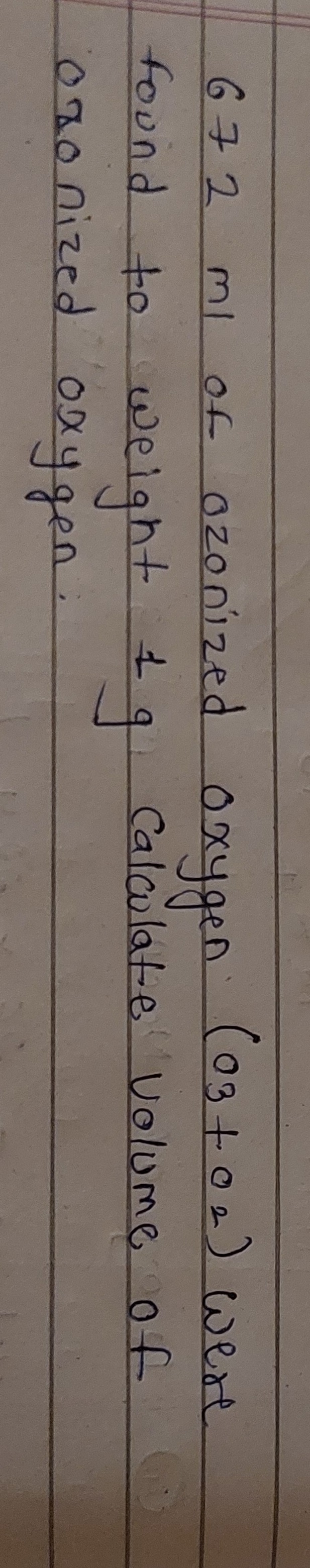
Answer
56 ml
Explanation
Solution
Let x be the volume (in ml) of ozone (O3) in the mixture. Then, the volume of oxygen (O2) is 672−x ml.
At STP, 22.4L=22400ml is the molar volume.
- Molar mass of O3=48g
- Molar mass of O2=32g
The mass contributed by ozone is:
Mass of O3=22400x×48gThe mass contributed by oxygen is:
Mass of O2=22400(672−x)×32gThe total mass is given as 1 g, so:
2240048x+2240032(672−x)=1Multiply through by 22400:
48x+32(672−x)=22400Simplify:
48x+21504−32x=22400 16x+21504=22400 16x=22400−21504=896 x=16896=56mlThus, the volume of ozone in the ozonized oxygen is 56 ml.
