Question
Question: Which of the following cannot reduce the acidified solution of KMnO4?...
Which of the following cannot reduce the acidified solution of KMnO4?
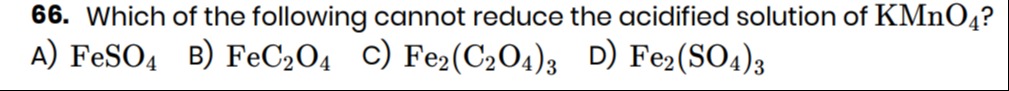
A
FeSO4
B
FeC2O4
C
Fe2(C2O4)3
D
Fe2(SO4)3
Answer
Fe2(SO4)3
Explanation
Solution
KMnO4 is a strong oxidizing agent. For a substance to reduce acidified KMnO4, it must contain an element that can be oxidized to a higher oxidation state.
- FeSO4: Fe is in +2 state, can be oxidized to +3.
- FeC2O4: Fe is in +2 state, can be oxidized to +3. C in C2O4^2- is in +3 state, can be oxidized to +4 (in CO2).
- Fe2(C2O4)3: Fe is in +3 state, cannot be oxidized further. C in C2O4^2- is in +3 state, can be oxidized to +4 (in CO2).
- Fe2(SO4)3: Fe is in +3 state, cannot be oxidized further. S in SO4^2- is in +6 state, cannot be oxidized further.
Thus, Fe2(SO4)3 cannot reduce acidified KMnO4.
