Question
Question: 6.4 litres of aq. NaCl is electrolysed for 1 minute 4 sec using 19.3 amperes (current efficiency is ...
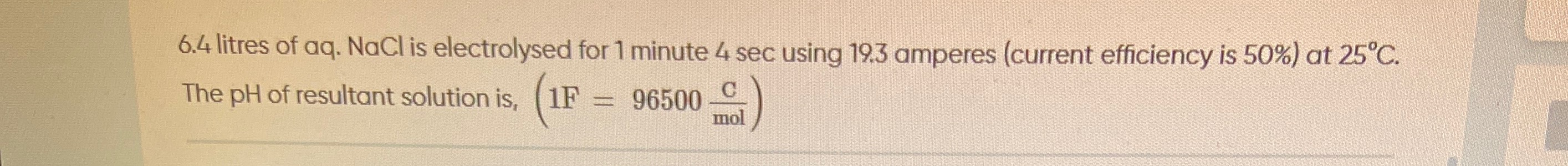
6.4 litres of aq. NaCl is electrolysed for 1 minute 4 sec using 19.3 amperes (current efficiency is 50%) at 25°C. The pH of resultant solution is, (1F=96500molC)
Answer
11
Explanation
Solution
To determine the pH of the resultant solution after electrolysis of aqueous NaCl, we follow these steps:
-
Identify Electrode Reactions: During the electrolysis of aqueous NaCl, the following reactions occur:
- At Cathode (Reduction): Water is reduced in preference to Na⁺ ions due to its higher reduction potential. 2H2O(l)+2e−→H2(g)+2OH−(aq)
- At Anode (Oxidation): Chloride ions are oxidized in preference to water due to overpotential effects for oxygen formation. 2Cl−(aq)→Cl2(g)+2e− The production of OH− ions at the cathode increases the pH of the solution.
-
Calculate Total Charge Passed (Q_total):
- Time, t=1 minute 4 seconds=60+4=64 s
- Current, I=19.3 A
- Total charge, Qtotal=I×t=19.3 A×64 s=1235.2 C
-
Calculate Actual Charge Used (Q_actual) considering Current Efficiency:
- Current efficiency = 50% = 0.50
- Actual charge used, Qactual=Qtotal×efficiency=1235.2 C×0.50=617.6 C
-
Calculate Moles of Electrons (n_e):
- Using Faraday's constant (1F=96500 C/mol e−):
- Moles of electrons, ne=FQactual=96500 C/mol617.6 C=0.0064 mol
-
Determine Moles of OH⁻ Produced:
- From the cathode reaction, 2H2O(l)+2e−→H2(g)+2OH−(aq), it is clear that 2 moles of electrons produce 2 moles of OH− ions.
- Therefore, moles of OH− produced = moles of electrons = 0.0064 mol
-
Calculate Concentration of OH⁻ Ions:
- Volume of solution = 6.4 litres
- Concentration of OH−, [OH−]=Volume of solutionMoles of OH−=6.4 L0.0064 mol=0.001 M=1×10−3 M
-
Calculate pOH:
- pOH = −log[OH−]=−log(1×10−3)=3
-
Calculate pH:
- At 25°C, pH+pOH=14
- pH = 14−pOH=14−3=11
The pH of the resultant solution is 11.
