Question
Question: Which of the following equations has AfH° and ∆Η° same?...
Which of the following equations has AfH° and ∆Η° same?
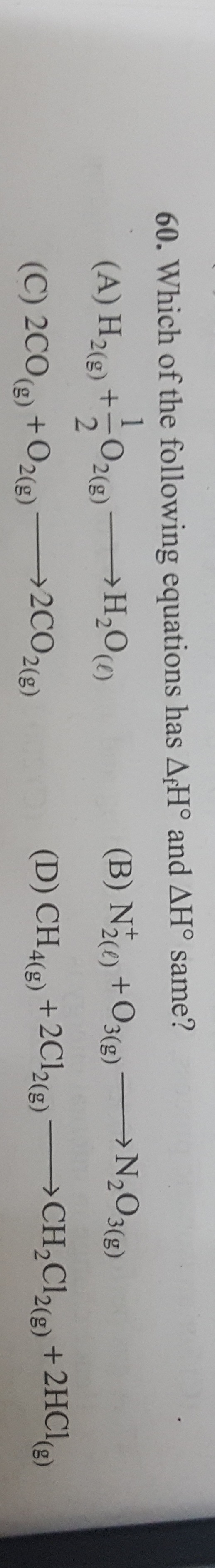
H2(g) + 21O2(g) →H2O(l)
N2(l) + O3(g) →N2O3(g)
2CO(g) + O2(g) → 2CO2(g)
CH4(g) + 2Cl2(g) →CH2Cl2(g) + 2HCl(g)
H2(g) + 21O2(g) →H2O(l)
Solution
For a reaction, if all reactants are in their standard elemental states, then the enthalpy change (ΔH°) of the reaction is the standard enthalpy of formation (Δ_fH°) of the product (or products) formed. In option (A):
H2(g)+21O2(g)→H2O(l)Both hydrogen and oxygen are in their standard states. Therefore, the reaction directly represents the formation of water from its elemental constituents, and hence ΔH° is exactly the standard enthalpy of formation of water.
The other options do not have all reactants in their standard states (or the reaction isn’t the direct formation reaction for a compound), and thus the enthalpy change does not equate to the standard enthalpy of formation.
