Question
Question: Iron fillings and water were placed in a 5 L vessel and sealed. The tank was heated to 1000°C. Upon ...
Iron fillings and water were placed in a 5 L vessel and sealed. The tank was heated to 1000°C. Upon analysis, the tank was found to contain 1.2 g of H₂(g) and 54.0 g of H₂O(g). If the reaction is represented as 3Fe(s)+4H2O(g)⇌Fe3O4(s)+4H2(g), then the value of equilibrium constant is
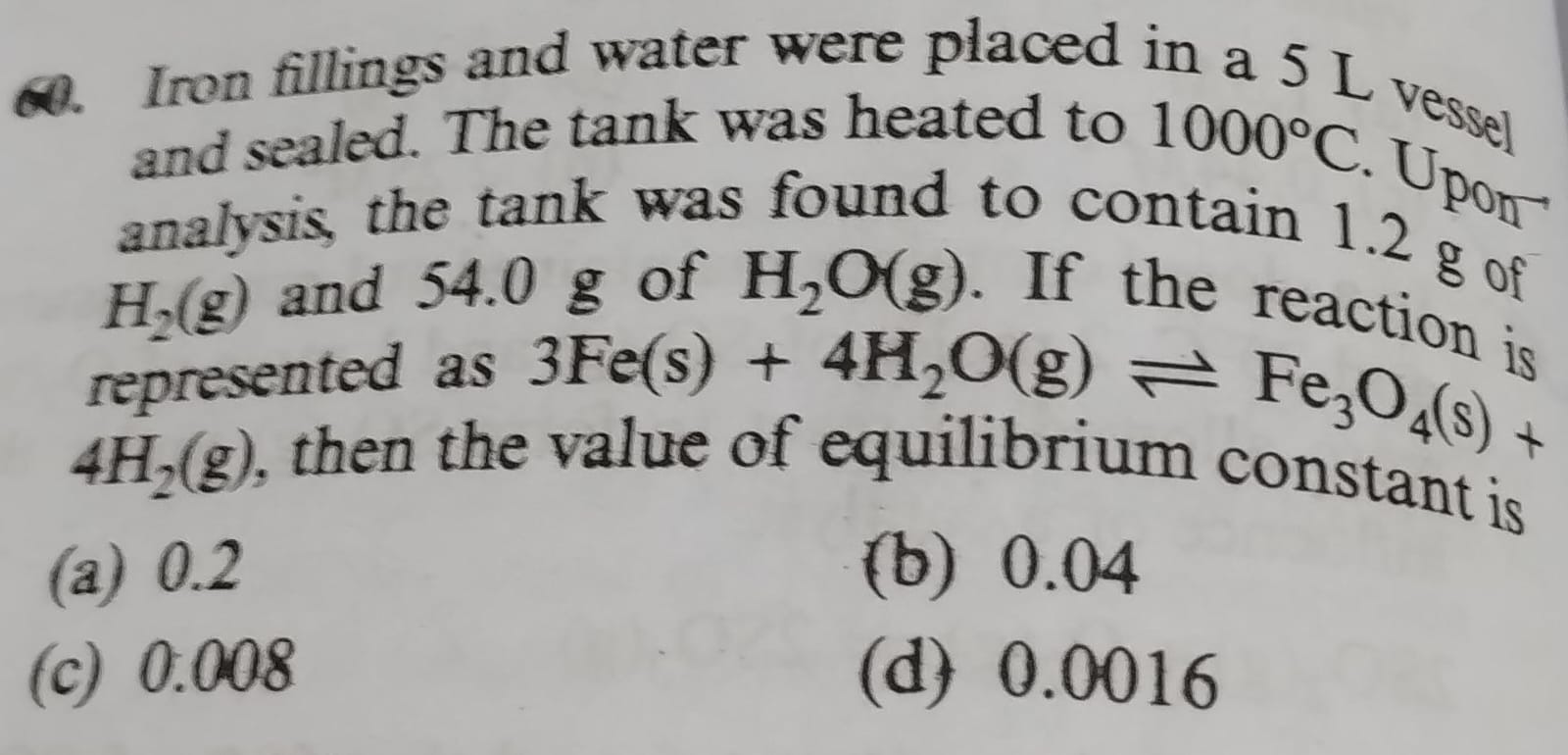
A
0.2
B
0.04
C
0.008
D
0.0016
Answer
0.0016
Explanation
Solution
The equilibrium constant expression is Kc=[H2O]4[H2]4. Moles of H2=2 g/mol1.2 g=0.6 mol. Moles of H2O=18 g/mol54.0 g=3.0 mol. Concentration of H2=5 L0.6 mol=0.12 M. Concentration of H2O=5 L3.0 mol=0.6 M. Kc=(0.60.12)4=(51)4=6251=0.0016.
