Question
Question: How many diastereomers exist for the compound below? ...
How many diastereomers exist for the compound below?
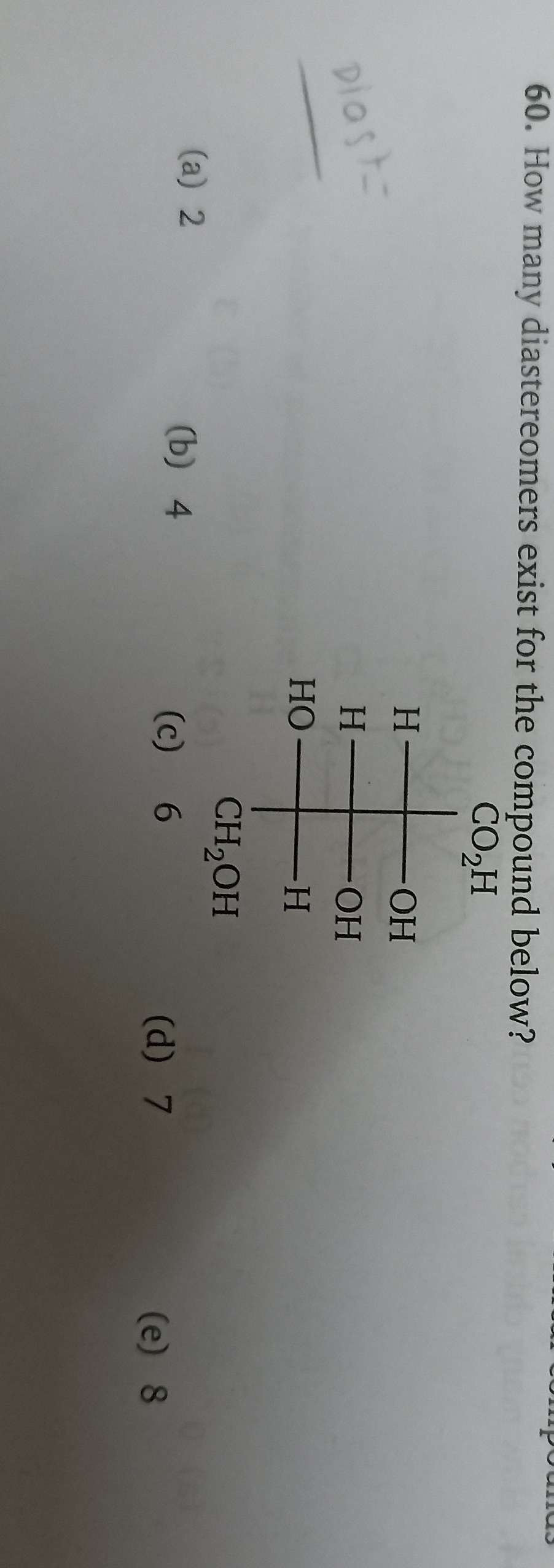
2
4
6
7
8
6
Solution
To determine the number of diastereomers for the given compound, we need to follow these steps:
-
Identify the chiral centers: The given compound is represented by a Fischer projection:
Let's number the carbons from the top, with CO2H as C1.
- C1 (CO2H) is not chiral as it has a double bond to oxygen and an OH group.
- C2 is bonded to CO2H, OH, H, and the rest of the chain (C3-C4-CH2OH). All four groups are different, so C2 is a chiral center.
- C3 is bonded to C2, OH, H, and the rest of the chain (C4-CH2OH). All four groups are different, so C3 is a chiral center.
- C4 is bonded to C3, OH, H, and CH2OH. All four groups are different, so C4 is a chiral center.
- C5 (CH2OH) is not chiral as it has two hydrogen atoms attached to it.
Thus, there are 3 chiral centers (C2, C3, and C4). Let n = 3.
-
Determine if meso compounds are possible: A meso compound is an achiral compound that possesses chiral centers due to an internal plane of symmetry or a center of inversion. Meso compounds typically arise in molecules with an even number of chiral centers and identical end groups.
In the given compound, the two end groups are CO2H (top) and CH2OH (bottom). Since these two groups are different, the molecule is unsymmetrical. Therefore, no meso form is possible for this compound.
-
Calculate the total number of stereoisomers: For a compound with 'n' chiral centers and no meso forms, the total number of possible stereoisomers is 2^n. In this case, n = 3, so the total number of stereoisomers = 2^3 = 8.
-
Calculate the number of diastereomers: Diastereomers are stereoisomers that are not mirror images of each other. If a compound is chiral, it will have one enantiomer (its non-superimposable mirror image). All other stereoisomers that are not the compound itself and not its enantiomer are its diastereomers.
For a specific stereoisomer, the number of its diastereomers is given by: Number of diastereomers = (Total number of stereoisomers) - 1 (the compound itself) - 1 (its enantiomer) Number of diastereomers = 2^n - 2 Substituting n = 3: Number of diastereomers = 2^3 - 2 = 8 - 2 = 6.
Therefore, there are 6 diastereomers for the given compound.
