Question
Question: Which of the following orders is incorrect? (X=F/Cl)...
Which of the following orders is incorrect? (X=F/Cl)
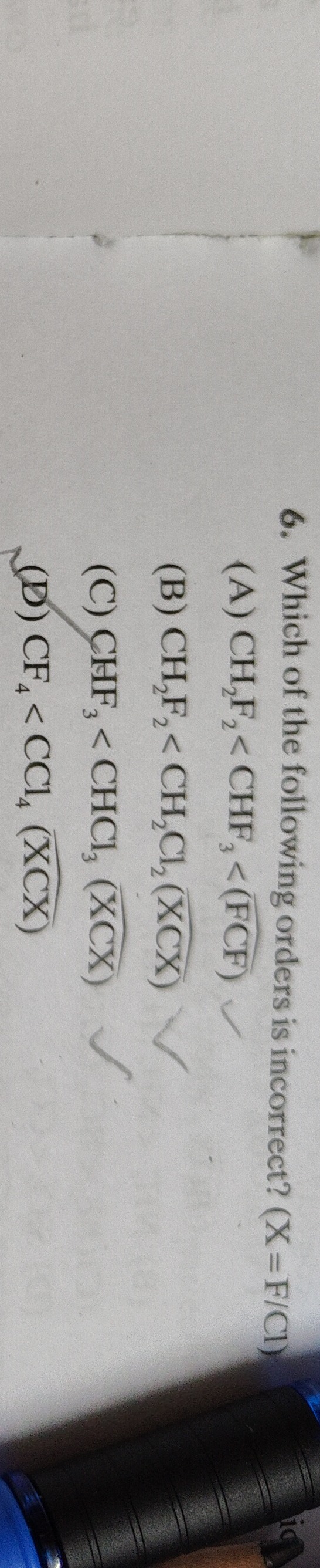
CH₂F₂< CHF₃ <(FCF) ✓
CH₂F₂ < CH₂Cl₂ (XCX)
CHF₃ < CHCl₃ (XCX) ✓
CF₄ < CCl₄ (XCX)
D
Solution
The question asks us to identify the incorrect order among the given options, comparing X-C-X bond angles where X is F or Cl. All molecules (CH₂F₂, CHF₃, CF₄, CH₂Cl₂, CHCl₃, CCl₄) have a central carbon atom that is sp³ hybridized, implying an ideal tetrahedral bond angle of 109.5°. However, deviations occur due to differences in electronegativity and steric effects of the substituents.
Let's analyze each option using known experimental bond angle values and general trends:
General Trends for Bond Angles:
-
Electronegativity of Peripheral Atoms: If the peripheral atoms are more electronegative, they pull electron density away from the central atom. This reduces the electron-electron repulsion between the bonding pairs near the central atom, leading to a decrease in the bond angle.
- Example: F is more electronegative than Cl. So, generally, F-C-F angles are expected to be smaller than Cl-C-Cl angles for similar substitution patterns.
-
Effect of Number of Electronegative Atoms / Steric Effects: In substituted methanes, the presence of less electronegative and relatively bulkier H atoms can influence the angles. C-H bonds have electron density closer to carbon than C-X bonds (where X is electronegative), leading to greater repulsion from C-H bonds.
Let's use the approximate experimental bond angles for these molecules:
- CH₂F₂: F-C-F angle ≈ 108.3°
- CHF₃: F-C-F angle ≈ 108.6°
- CF₄: F-C-F angle = 109.5° (ideal tetrahedral due to symmetry)
- CH₂Cl₂: Cl-C-Cl angle ≈ 111.8°
- CHCl₃: Cl-C-Cl angle ≈ 110.5°
- CCl₄: Cl-C-Cl angle = 109.5° (ideal tetrahedral due to symmetry)
Now, let's evaluate each option:
(A) CH₂F₂ < CHF₃ < CF₄ (F-C-F) Comparing the F-C-F angles:
- CH₂F₂ (F-C-F) ≈ 108.3°
- CHF₃ (F-C-F) ≈ 108.6°
- CF₄ (F-C-F) = 109.5° The order is 108.3° < 108.6° < 109.5°, which is correct. This trend can be explained by the decreasing influence of the C-H bonds (which tend to compress the F-C-F angle) as H atoms are replaced by F atoms, leading to the ideal tetrahedral angle in CF₄.
(B) CH₂F₂ < CH₂Cl₂ (XCX) Comparing the X-C-X angles:
- CH₂F₂ (F-C-F) ≈ 108.3°
- CH₂Cl₂ (Cl-C-Cl) ≈ 111.8° The order is 108.3° < 111.8°, which is correct. This is consistent with the electronegativity rule: F is more electronegative than Cl, so F-C-F angle is smaller than Cl-C-Cl angle.
(C) CHF₃ < CHCl₃ (XCX) Comparing the X-C-X angles:
- CHF₃ (F-C-F) ≈ 108.6°
- CHCl₃ (Cl-C-Cl) ≈ 110.5° The order is 108.6° < 110.5°, which is correct. This is also consistent with the electronegativity rule: F-C-F angle is smaller than Cl-C-Cl angle.
(D) CF₄ < CCl₄ (XCX) Comparing the X-C-X angles:
- CF₄ (F-C-F) = 109.5°
- CCl₄ (Cl-C-Cl) = 109.5° Both CF₄ and CCl₄ are perfectly symmetrical tetrahedral molecules. In such molecules, all bond angles are ideally 109.5°. Therefore, F-C-F angle in CF₄ is equal to Cl-C-Cl angle in CCl₄. The order "CF₄ < CCl₄" is incorrect as they are equal.
The question asks for the incorrect order. Based on our analysis, option (D) is incorrect.
