Question
Question: Which of the following having S-O-S linkage-...
Which of the following having S-O-S linkage-
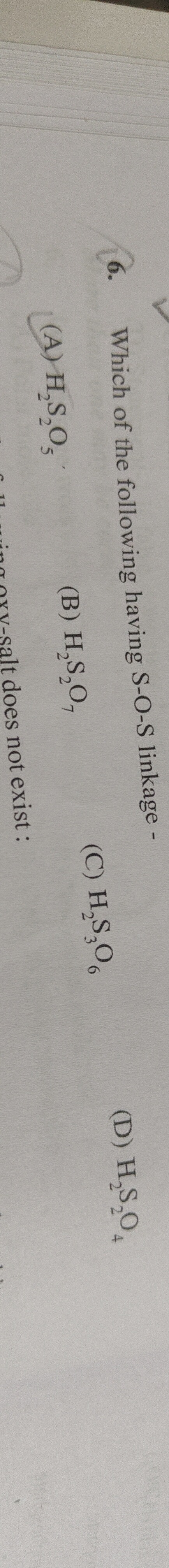
H2S2O5
H2S2O7
H2S3O6
H2S2O4
H2S2O7
Solution
To determine which of the given compounds has an S-O-S linkage, we need to analyze the molecular structure of each option.
-
H₂S₂O₅ (Disulfurous acid or Pyrosulfurous acid):
The structure contains an S-O-S linkage: HO−S(=O)−O−S(=O)−OH -
H₂S₂O₇ (Disulfuric acid or Pyrosulfuric acid):
The structure contains an S-O-S linkage: HO−S(=O)₂−O−S(=O)₂−OH -
H₂S₃O₆ (Trithionic acid):
The structure contains an S-S-S linkage, not an S-O-S linkage: HO−S(=O)₂−S−S(=O)₂−OH -
H₂S₂O₄ (Dithionous acid):
The structure contains an S-S linkage, not an S-O-S linkage: HO−S(=O)−S(=O)−OH
Both H₂S₂O₅ and H₂S₂O₇ contain an S-O-S linkage. H₂S₂O₇ (pyrosulfuric acid) is a very well-known and stable compound with a definitive S-O-S linkage.
