Question
Question: The compound which shows highest reactivity towards nucleophilic displacement reaction is ...
The compound which shows highest reactivity towards nucleophilic displacement reaction is
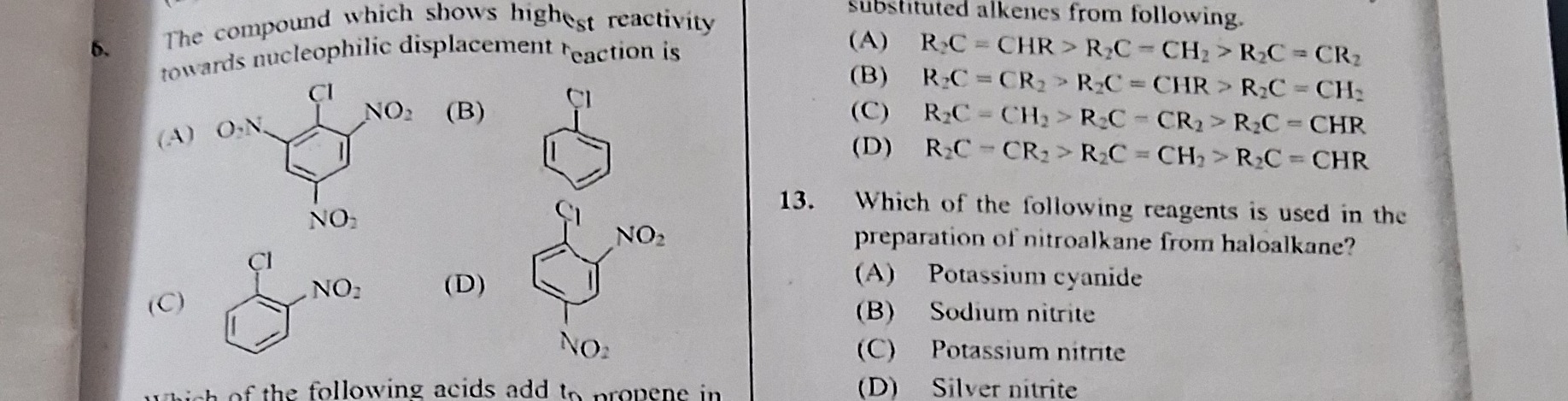
R2C=CHR>R2C=CH2>R2C=CR2
R2C=CR2>R2C=CHR>R2C=CH2
R2C=CH2>R2C=CR2>R2C=CHR
R2C=CR2>R2C=CH2>R2C=CHR
Option (A) as it has nitro groups at the ortho and para positions relative to the chlorine atom, which stabilizes the Meisenheimer complex during nucleophilic aromatic substitution.
Solution
For nucleophilic aromatic substitution, the presence of strong –I/–M groups (like –NO₂) at the ortho and/or para positions relative to the leaving group (–Cl) stabilizes the Meisenheimer complex.
A chlorine attached to a benzene ring having nitro groups both at ortho and para positions (i.e. 2‑ and 4‑positions relative to chlorine) is much more activated than one with nitro groups elsewhere (e.g. meta) or having only one nitro group.
Thus, the compound with –NO₂ at the ortho and para positions relative to –Cl (option (A) in the figure, which is the same as option (D)) shows the highest reactivity.
