Question
Question: The compound which shows highest reactivity towards nucleophilic displacement reaction is ...
The compound which shows highest reactivity towards nucleophilic displacement reaction is
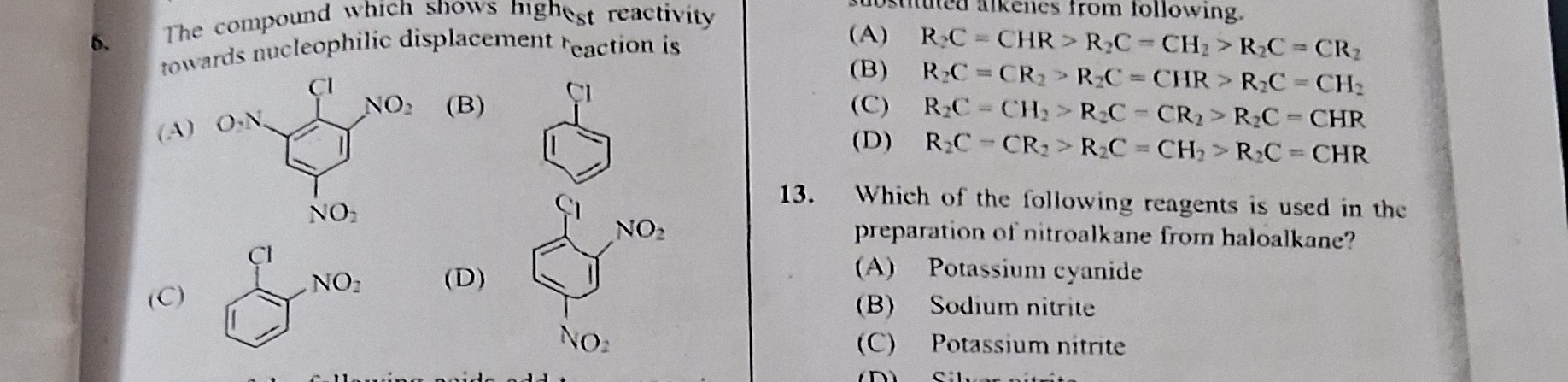
A
R2C=CHR>R2C=CH2>R2C=CR2
B
R2C=CR2>R2C=CHR>R2C=CH2
C
R2C=CH2>R2C=CR2>R2C=CHR
D
R2C−CR2>R2C=CH2>R2C=CHR
Answer
Option D is correct.
Explanation
Solution
For nucleophilic aromatic substitution, the presence of strong –I/–M groups (like –NO₂) at the ortho or para positions to the leaving group (Cl) greatly enhances reactivity by stabilizing the intermediate σ‐complex.
- In structure (A): Cl is at position 2 with NO₂ groups at positions 1 (ortho) and 4 (meta).
- In structure (B) and (C): Cl is at position 1 with a single NO₂ at position 2 (ortho).
- In structure (D): Cl is at position 1 with NO₂ groups at positions 2 (ortho) and 4 (para).
Thus, (D) has both ortho and para –NO₂ groups relative to the leaving group, providing the best stabilization of the intermediate; hence, it shows the highest reactivity.
