Question
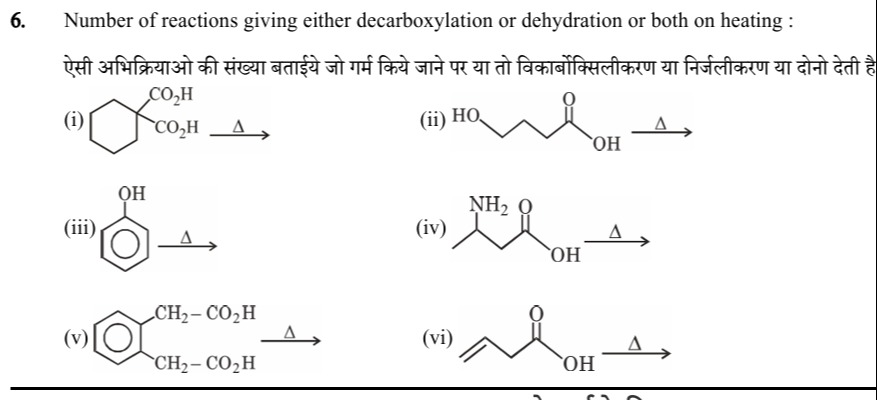
Question: Number of reactions giving either decarboxylation or dehydration or both on heating : ऐसी अभिक्रियाओ...
Number of reactions giving either decarboxylation or dehydration or both on heating : ऐसी अभिक्रियाओ की संख्या बताईये जो गर्म किये जाने पर या तो विकार्बोक्सिलीकरण या निर्जलीकरण या दोनो देती है।
5
Solution
(i) The geminal di‐acid on the cyclohexane ring loses CO₂ on heating → decarboxylation.
(ii) The molecule with –OH at one end and –COOH at the other undergoes ring closure by loss of H₂O → dehydration.
(iii) The benzene–OH system has no second group to eliminate water with or to lose CO₂ → no reaction.
(iv) The amino–acid type structure decarboxylates on heating → decarboxylation.
(v) The benzene with two –CH₂CO₂H groups (ortho) forms an anhydride by eliminating water → dehydration.
(vi) The structure having a β–hydroxy acid group loses water → dehydration.
Thus, out of six, reactions (i), (ii), (iv), (v) and (vi) involve either decarboxylation or dehydration (or both).
