Question
Question: If 0.5 M methanol undergo self dissociation like $CH_3OH \rightleftharpoons CH_3O^- + H^+$ & if conc...
If 0.5 M methanol undergo self dissociation like CH3OH⇌CH3O−+H+ & if concentration of H+ is 2.5 × 10−4 M then calculate % dissociation of methanol.
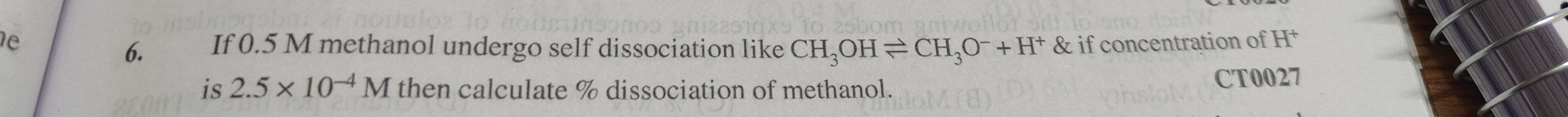
0.05%
Solution
The self-dissociation of methanol can be represented by the following equilibrium:
CH3OH⇌CH3O−+H+
Let's set up an ICE (Initial, Change, Equilibrium) table for the dissociation:
Initial concentration of CH3OH=0.5 M
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| CH3OH | 0.5 | −x | 0.5−x |
| CH3O− | 0 | +x | x |
| H+ | 0 | +x | x |
We are given that the concentration of H+ at equilibrium is 2.5×10−4 M. From the ICE table, at equilibrium, [H+]=x. Therefore, x=2.5×10−4 M.
The percentage dissociation (α) is calculated as the ratio of the concentration of methanol that dissociated to its initial concentration, multiplied by 100.
% dissociation=initial concentrationconcentration dissociated×100%
In this case, the concentration dissociated is x.
% dissociation=0.5x×100%
Substitute the value of x:
% dissociation=0.52.5×10−4×100%
% dissociation=0.52.5×10−4×100%
% dissociation=5×10−4×102%
% dissociation=5×10−2%
% dissociation=0.05%
The percentage dissociation of methanol is 0.05%.
