Question
Question: Identify the product 'B' in the following reaction sequence. $C_2H_5-Br \xrightarrow[Dry ether]{Mg}...
Identify the product 'B' in the following reaction sequence.
C2H5−BrMgDryetherACH3OHB
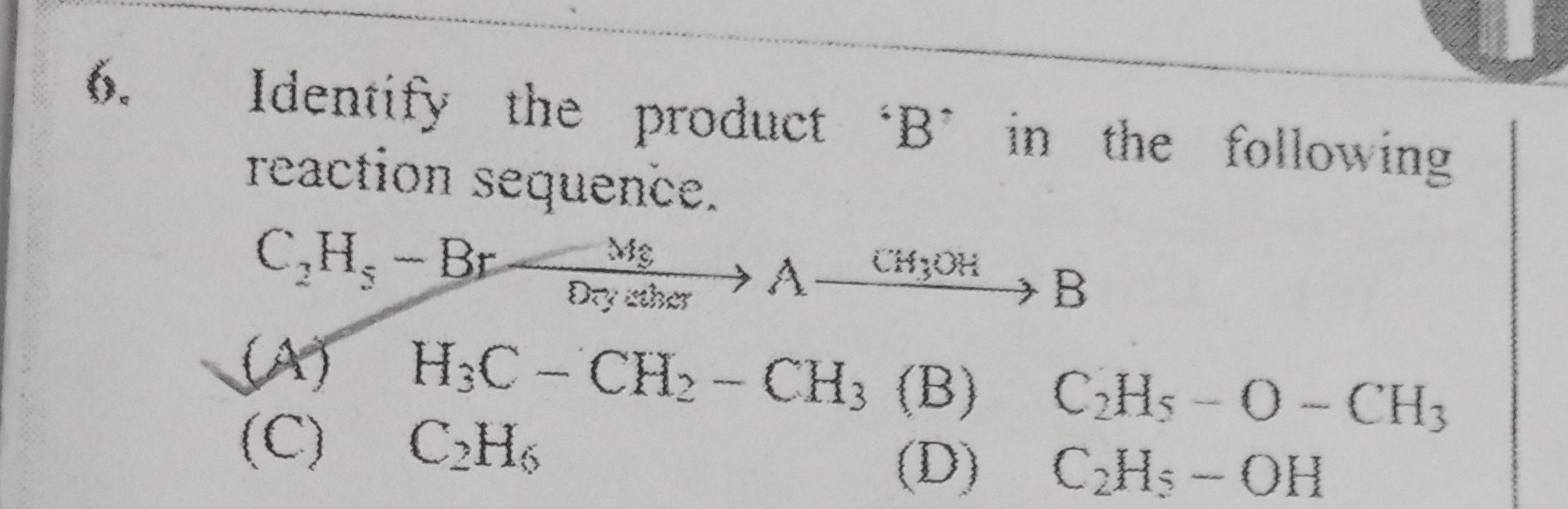
A
H3C−CH2−CH3
B
C2H5−O−CH3
C
C2H6
D
C2H5−OH
Answer
C2H6
Explanation
Solution
-
Formation of Grignard Reagent:
C2H5-Br+Mgdry etherC2H5MgBr(Product A) -
Reaction with Methanol:
The Grignard reagent, being strongly basic, reacts with methanol (CH3OH) by abstracting a proton:
C2H5MgBr+CH3OH→C2H6+MgBrOCH3Thus, product B is ethane (C2H6).
