Question
Question: Analyze the stability of the following carbocations:...
Analyze the stability of the following carbocations:
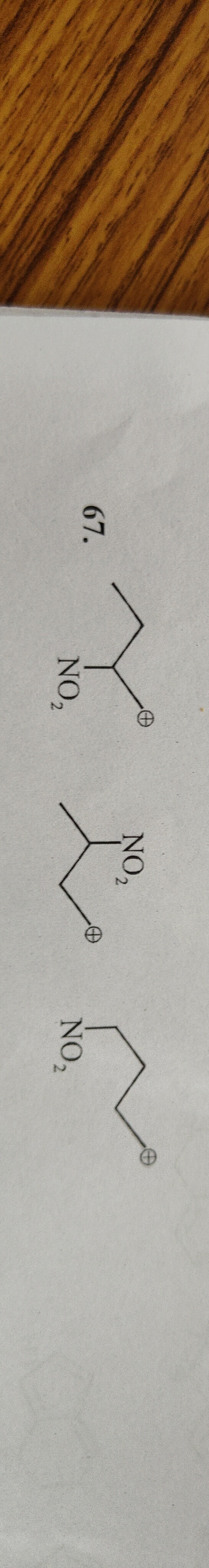
Structure 1: CH3−C+H−CH2−NO2
Structure 2: CH3−C+H−NO2CH−CH3
Structure 3: CH3−CH2−C+H−NO2
Structure 2 is the most stable carbocation.
Solution
Carbocation stability is influenced by inductive effects, hyperconjugation, and resonance. Electron-donating groups stabilize carbocations, while electron-withdrawing groups destabilize them. The nitro group (-NO2) is a strong electron-withdrawing group. Its destabilizing effect is strongest at the alpha position and weaker at the beta position. Secondary carbocations are more stable than primary carbocations due to better stabilization by alkyl groups.
- Structure 1: Primary carbocation with a beta-nitro group. Stabilized by one alkyl group and hyperconjugation, destabilized by the beta-nitro group.
- Structure 2: Secondary carbocation with a beta-nitro group. Stabilized by two alkyl groups and hyperconjugation, destabilized by the beta-nitro group. This offers better alkyl stabilization than Structure 1.
- Structure 3: Secondary carbocation with an alpha-nitro group. Stabilized by one alkyl group and hyperconjugation, but significantly destabilized by the alpha-nitro group due to its proximity and strong electron-withdrawing effect.
Comparing them:
- Structure 3 is the least stable due to the alpha-nitro group.
- Structure 2 is more stable than Structure 1 because it is a secondary carbocation (better alkyl stabilization) with a beta-nitro group.
- Structure 1 is less stable than Structure 2 because it is a primary carbocation.
Therefore, the order of stability is: Structure 2 > Structure 1 > Structure 3.
Assuming the question asks for the most stable carbocation, the answer is Structure 2.
