Question
Question: (a) (Number of grignard reagent consumed) = x (b) $\xrightarrow[KMnO_4]{\text{dil. alk.}}$ Produ...
(a) (Number of grignard reagent consumed) = x
(b) dil. alk.KMnO4 Product ; (Number of possible stereoisomers of major product) = y
then value of x + y ?
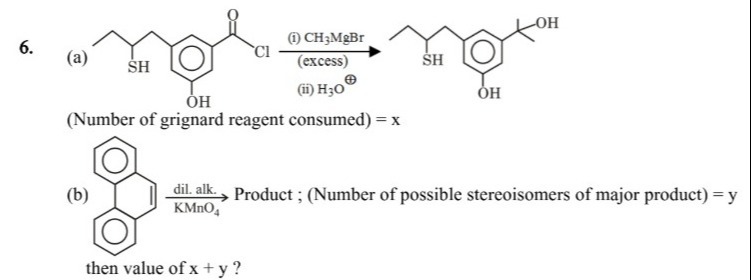
6
Solution
Part (a): Number of Grignard reagent consumed (x)
The reactant molecule has three types of functional groups that can react with a Grignard reagent (CH3MgBr):
-
Acyl chloride (-COCl): Acyl chlorides react with Grignard reagents in two steps when the Grignard reagent is in excess.
-
First, one mole of CH3MgBr adds to the carbonyl carbon, and the chloride ion leaves, forming a ketone.
R-COCl + CH3MgBr → R-CO-CH3 + MgClBr
-
Second, since the Grignard reagent is in excess, the newly formed ketone reacts with another mole of CH3MgBr to form a tertiary alkoxide, which upon hydrolysis gives a tertiary alcohol.
R-CO-CH3 + CH3MgBr → R-C(OMgBr)(CH3)2
R-C(OMgBr)(CH3)2 + H3O+ → R-C(OH)(CH3)2 + MgBr(OH)
Therefore, 2 moles of CH3MgBr are consumed for the conversion of the acyl chloride group to a tertiary alcohol.
-
-
Phenolic hydroxyl (-OH): Phenolic protons are acidic and react with Grignard reagents, which are strong bases.
Ar-OH + CH3MgBr → Ar-OMgBr + CH4
This reaction consumes 1 mole of CH3MgBr. During the subsequent acidic workup (H3O+), the Ar-OMgBr is protonated back to Ar-OH.
-
Thiol (-SH): Thiol protons are also acidic and react with Grignard reagents.
R-SH + CH3MgBr → R-SMgBr + CH4
This reaction consumes 1 mole of CH3MgBr. During the subsequent acidic workup (H3O+), the R-SMgBr is protonated back to R-SH.
Total number of Grignard reagent (CH3MgBr) moles consumed (x) = 2 (for acyl chloride) + 1 (for phenolic -OH) + 1 (for thiol -SH) = 4. So, x = 4.
Part (b): Number of possible stereoisomers of major product (y)
The reactant molecule is Phenanthrene. The reaction is with dil. alk. KMnO4, which is a reagent for syn-dihydroxylation (Baeyer's test).
Phenanthrene is an aromatic compound. Aromatic compounds are generally resistant to oxidation by mild reagents like dilute alkaline KMnO4. However, among the double bonds in phenanthrene, the 9,10-double bond has the highest bond order and is the most reactive towards addition reactions. If it reacts, it undergoes dihydroxylation.
Assuming the 9,10-double bond of phenanthrene reacts to form a diol:
The product will be 9,10-dihydro-9,10-phenanthrenediol.
Structure of 9,10-dihydro-9,10-phenanthrenediol: The carbons at positions 9 and 10 become sp3 hybridized and each bears a hydroxyl group and a hydrogen atom (implied). These two carbons (C9 and C10) are chiral centers.
Syn-dihydroxylation adds two -OH groups to the same face of the double bond. Since the starting material (phenanthrene) is achiral, the syn-addition will lead to a racemic mixture of enantiomers. Let's consider the two chiral centers at C9 and C10. Due to the rigid, non-planar nature of the 9,10-dihydrophenanthrene skeleton (the central ring adopts a boat conformation), the two chiral centers are not identical in a way that would lead to a meso compound from syn-addition. Syn-addition across the 9,10-double bond of phenanthrene will produce two stereoisomers, which are a pair of enantiomers. For example, if the -OH groups are added from the "top" face, one specific enantiomer is formed (e.g., 9R,10R). If they are added from the "bottom" face, its enantiomer is formed (e.g., 9S,10S). These are the only two possible stereoisomers formed by syn-addition.
So, the number of possible stereoisomers of the major product (y) = 2.
Value of x + y x = 4 y = 2 x + y = 4 + 2 = 6.
