Question
Question: \(5g\) of water rises in the bore of the capillary tube when it is dipped in water. If the radius of...
5g of water rises in the bore of the capillary tube when it is dipped in water. If the radius of the bore capillary tube is doubled, the mass of water that rises in the capillary tube above the outside water level is
(A) 1.5g
(B) 10g
(C) 5g
(D) 15g
Solution
Hint We know that the pressure at a point A and B in the diagram is the same. The pressure drop when going downwards through the meniscus from the point A is R2T, where T is the surface tension of water and R is the radius of the meniscus. This pressure drop is compensated by the pressure of the water column. Once we balance these, we will get our required solution.
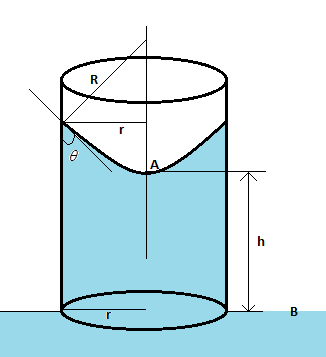
Complete Step by step solution The height of the water column is calculated from the equation ρgh=R2T, where ρ is the density of water, h is the height of the water column, g is the acceleration due to gravity, T is the surface tension of water, and R is the radius of the meniscus. Now from the figure, we get cosθ=rR, i.e. R=rcosθ such that the above equation becomes ρgh=rcosθ2T.
Also, we need to find the mass of the water column in terms of the height and radius of the cylinder. If M be the mass of the water column, then M=ρV=ρ(πr2h). Substituting h from the above equation, we get h=ρπr2M.
Replacing h, in the equation ρgh=rcosθ2T, we get ρπr2ρgM=rcosθ2T.
Cancelling terms and taking all constants on one side we get, rM=gcosθ2πT=const.
Now according to the given question, the mass of water in the capillary tube is 5grams, and the radius r gets doubled.
⇒rM=const.=r1M1=r2M2,
∴r15grams=2r1M2, or M2=10grams.
Therefore the correct option is an option (B).
Note Here we take the pressure drop as R2T and not R4T, since the water column has only a single layer. In the case of bubbles, the pressure difference between the concave and the convex sides is R4T. The concave side of a bubble has more pressure than the concave side.
