Question
Question: Select the best reaction sequence to accomplish the following transformation ...
Select the best reaction sequence to accomplish the following transformation
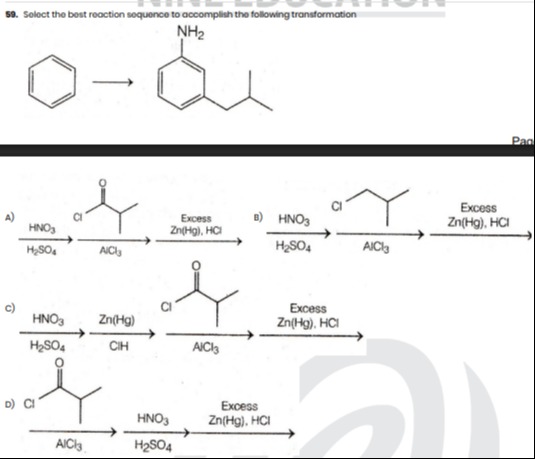
H2SO4HNO3 ⟶ AlCl3Cl ⟶ Zn(Hg),HClExcess ⟶
H2SO4HNO3 ⟶ AlCl3Cl ⟶ Zn(Hg),HClExcess ⟶
H2SO4HNO3 ⟶ ClHZn(Hg) ⟶ AlCl3Cl ⟶ Zn(Hg),HClExcess ⟶
AlCl3Cl ⟶ H2SO4HNO3 ⟶ Zn(Hg),HClExcess ⟶
D
Solution
The transformation requires converting benzene into 3-isobutyl-aniline. The key aspects are introducing an amino group and an isobutyl group, and ensuring they are in a meta-relationship on the benzene ring.
Let's analyze the directing effects of functional groups:
- Alkyl groups (-R) and Amino groups (-NH2) are ortho/para-directing and activating.
- Nitro groups (-NO2) and Acyl groups (-COR) are meta-directing and deactivating.
To achieve a meta-relationship between the final amino and isobutyl groups, at least one of the groups introduced early in the synthesis must be a meta-director when the second group is added.
Option D is the most chemically sound and practical sequence because the Friedel-Crafts reactions in options A, B, and C are problematic due to deactivation or complexation of the aromatic ring.
Option D follows the sequence:
- Friedel-Crafts Acylation: Benzene is acylated with isobutyryl chloride (Cl-CO-CH(CH3)2) using AlCl3 to form isobutyrophenone. This introduces the carbon chain.
- Nitration: The isobutyrophenone then undergoes nitration with HNO3/H2SO4. The acyl group (−COR) is a meta-director, so the nitro group (−NO2) adds to the meta position, establishing the desired meta-relationship.
- Reduction: The resulting 3-nitro-isobutyrophenone is treated with excess Zn(Hg), HCl (Clemmensen reduction conditions). This reagent simultaneously reduces the ketone to an alkane (converting the isobutyryl group to an isobutyl group) and the nitro group to an amino group, yielding 3-isobutyl-aniline.
