Question
Question: 58. $\text{CH}_3\text{-CH}_2\text{-C} \equiv \text{CH} \rightleftharpoons^{\text{A}}_{\text{B}} \tex...
- CH3-CH2-C≡CH⇌BACH3C≡C-CH3
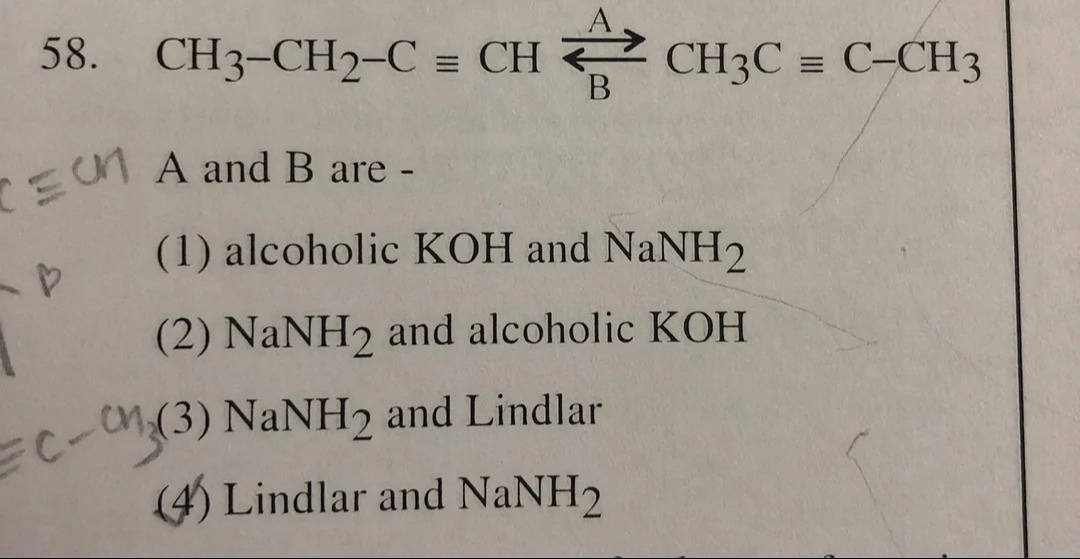
A
alcoholic KOH and NaNH2
B
NaNH2 and alcoholic KOH
C
NaNH2 and Lindlar
D
Lindlar and NaNH2
Answer
NaNH2 and alcoholic KOH
Explanation
Solution
The terminal alkyne (1‐butyne) is first deprotonated by a strong base. NaNH₂ (a very strong base) effectively removes the acidic terminal hydrogen, forming an acetylide ion that rearranges to form the internal alkyne (2‐butyne). In the reverse reaction, alcoholic KOH (a milder base) is used, thereby making the process reversible under controlled conditions.
