Question
Question: The major product in the following reaction is [Insert Reaction Scheme Image Here: A six-carbon cha...
The major product in the following reaction is
[Insert Reaction Scheme Image Here: A six-carbon chain with a methyl group at the second carbon and an iodine atom at the fourth carbon reacts with t-BuOH under heat.]
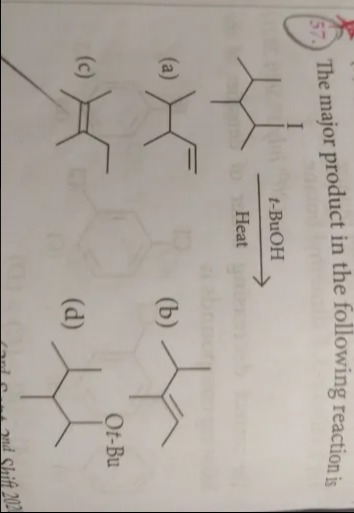
Product (a) - six-carbon chain with methyl group at the second carbon and a double bond between the fourth and fifth carbons.
Product (b) - six-carbon chain with methyl group at the second carbon and a double bond between the third and fourth carbons.
Product (c) - six-carbon chain with methyl group at the second carbon and double bonds between the third and fourth carbons and between the fifth and sixth carbons.
Product (d) - six-carbon chain with methyl group at the second carbon and a tert-butoxy group (Ot-Bu) at the fourth carbon.
Option (b)
Solution
The substrate, 4-iodo-2-methylhexane, undergoes E1 elimination when heated with t-BuOH. The iodide leaves, forming a carbocation at C4. Elimination occurs by removing a β-hydrogen from either C3 or C5. According to Zaitsev's rule, removal from C3 (adjacent to the more substituted center at C2) gives the more substituted, and therefore more stable, alkene. The major product has a double bond between C3 and C4.
