Question
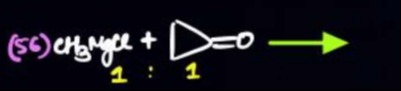
Question: $(56) \ CH_3MgCl + =O \longrightarrow$ $1:1$...
(56) CH3MgCl+=O⟶ 1:1
The product of the reaction is 1-methylcyclopropan-1-ol.
Solution
The reaction involves the addition of a Grignard reagent, methylmagnesium chloride (CH3MgCl), to a ketone, cyclopropanone. Grignard reagents are strong nucleophiles and react with carbonyl compounds to form alcohols after hydrolysis.
Reaction Mechanism:
-
Nucleophilic Attack: The methyl group (CH3−) from CH3MgCl acts as a nucleophile and attacks the electrophilic carbon of the carbonyl group (C=O) in cyclopropanone. This causes the π-bond of the carbonyl group to break, and the electrons move to the oxygen atom, forming an alkoxide intermediate.
\begin{center} $CH_3MgCl$ + \begin{tikzpicture} \draw (0,0) -- (1,0) -- (0.5,0.866) -- cycle; \node at (0.5,0.866) {C}; \node at (0,0) {CH$_2$}; \node at (1,0) {CH$_2$}; \draw (0.5,0.866) -- (0.5,1.5); \node at (0.5,1.7) {O}; \draw[dashed, ->] (0.5,1.5) -- (0.5,1.7); \node at (0.5,1.2) {\textbf{=}}; \end{tikzpicture} $\longrightarrow$ \begin{tikzpicture} \draw (0,0) -- (1,0) -- (0.5,0.866) -- cycle; \node at (0.5,0.866) {C}; \node at (0,0) {CH$_2$}; \node at (1,0) {CH$_2$}; \draw (0.5,0.866) -- (0.5,1.5); \node at (0.5,1.7) {O$^-$Mg$^+$Cl}; \draw (0.5,0.866) -- (1.2,0.866); \node at (1.4,0.866) {CH$_3$}; \end{tikzpicture} \end{center}(1-methylcyclopropyl)magnesium chloride (alkoxide intermediate)
-
Hydrolysis: The alkoxide intermediate is then protonated upon hydrolysis (typically by adding water and acid, H3O+) to form the corresponding alcohol.
\begin{center} \begin{tikzpicture} \draw (0,0) -- (1,0) -- (0.5,0.866) -- cycle; \node at (0.5,0.866) {C}; \node at (0,0) {CH$_2$}; \node at (1,0) {CH$_2$}; \draw (0.5,0.866) -- (0.5,1.5); \node at (0.5,1.7) {O$^-$Mg$^+$Cl}; \draw (0.5,0.866) -- (1.2,0.866); \node at (1.4,0.866) {CH$_3$}; \end{tikzpicture} $+ H_2O/H^+$ $\longrightarrow$ \begin{tikzpicture} \draw (0,0) -- (1,0) -- (0.5,0.866) -- cycle; \node at (0.5,0.866) {C}; \node at (0,0) {CH$_2$}; \node at (1,0) {CH$_2$}; \draw (0.5,0.866) -- (0.5,1.5); \node at (0.5,1.7) {OH}; \draw (0.5,0.866) -- (1.2,0.866); \node at (1.4,0.866) {CH$_3$}; \end{tikzpicture} \end{center}(1-methylcyclopropan-1-ol)
The final product is 1-methylcyclopropan-1-ol, which is a tertiary alcohol.
Explanation of the solution:
Methylmagnesium chloride (CH3MgCl) reacts with cyclopropanone via nucleophilic addition. The methyl group (CH3−) attacks the carbonyl carbon, forming an alkoxide intermediate. Subsequent hydrolysis of this intermediate yields 1-methylcyclopropan-1-ol.
