Question
Question: A quantity of 34 g sample of $BaO_2$ is heated to 1000 K in a closed and rigid evacuated vessel of 8...
A quantity of 34 g sample of BaO2 is heated to 1000 K in a closed and rigid evacuated vessel of 8.21 L capacity. What percentage of peroxide is converted into oxide? (Ba = 138)
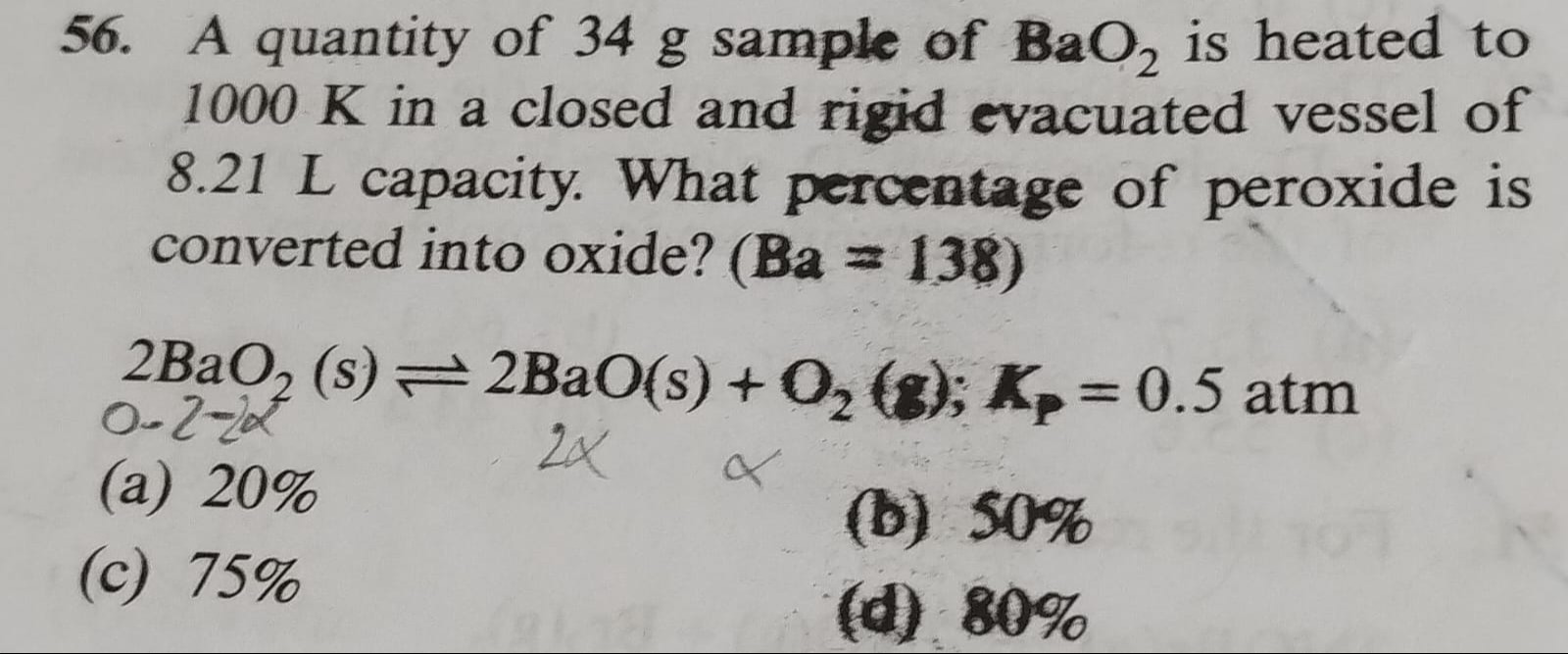
20%
50%
75%
80%
50%
Solution
The equilibrium constant Kp for the reaction 2BaO2(s)⇌2BaO(s)+O2(g) is given by Kp=PO2. Given Kp=0.5 atm, the partial pressure of O2 at equilibrium is PO2=0.5 atm. Using the ideal gas law, PV=nRT, we calculate the moles of O2 produced at equilibrium: nO2=RTPO2V=(0.0821 L atm/mol K)(1000 K)(0.5 atm)(8.21 L)=0.05 mol. The molar mass of BaO2 is 138+2×16=170 g/mol. The initial mass of BaO2 is 34 g, so the initial moles are: Initial moles of BaO2=170 g/mol34 g=0.2 mol. From the stoichiometry of the reaction (2BaO2→2BaO+O2), 2 moles of BaO2 decompose to produce 1 mole of O2. Therefore, if 0.05 mol of O2 is produced, the moles of BaO2 reacted are 2×0.05=0.1 mol. The percentage conversion of BaO2 is calculated as: Percentage conversion = initial moles of BaO2moles of BaO2 reacted×100%=0.2 mol0.1 mol×100%=50%.
