Question
Question: At 127°C and 1 atm pressure, PCl₅(g) is partially dissociated into PCl₂(g) and Cl₂(g) as PCl₅(g)⇌PCl...
At 127°C and 1 atm pressure, PCl₅(g) is partially dissociated into PCl₂(g) and Cl₂(g) as PCl₅(g)⇌PCl₂(g) + Cl₂(g). The density of the equilibrium mixture is 3.5 g/L. Percentage dissociation of PCl₅ is (R=0.08 L-atm/K-mol, P = 31, Cl = 35.5)
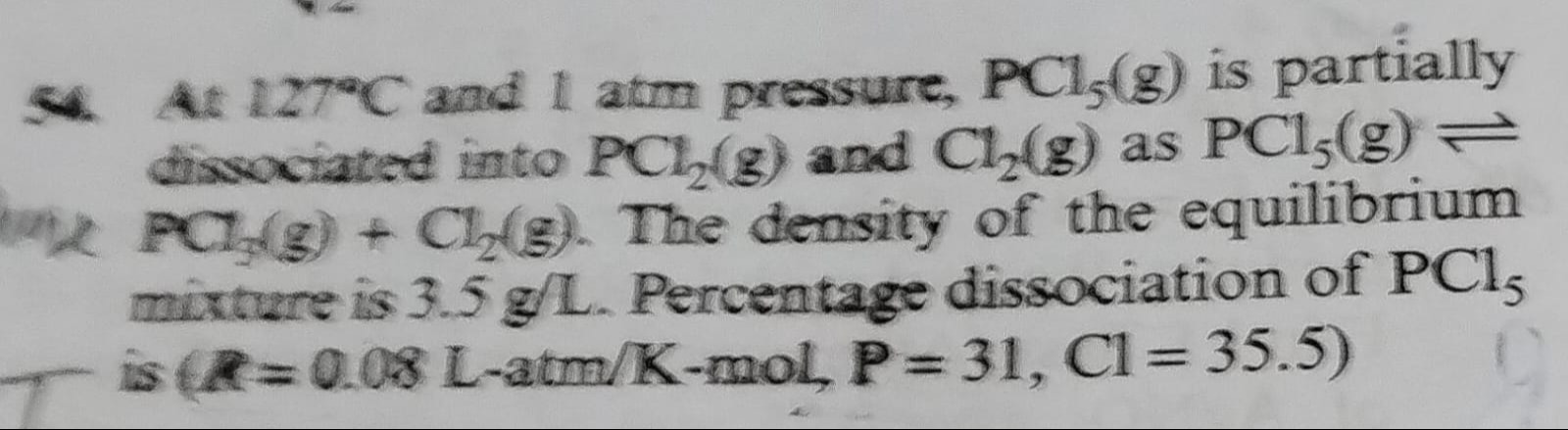
Answer
86.16%
Explanation
Solution
-
Molar mass of PCl₅ (M0): M0=31+5×35.5=208.5 g/mol
-
Molar mass of the equilibrium mixture (Mmix): Using the ideal gas law, ρ=RTPM, so Mmix=PρRT. Mmix=1 atm(3.5 g/L)×(0.08 L-atm/K-mol)×(127+273 K)=112 g/mol
-
Degree of dissociation (α): For PCl₅(g) ⇌ PCl₃(g) + Cl₂(g), n=2. The relation is Mmix=1+(n−1)αM0. 112=1+α208.5 1+α=112208.5≈1.8616 α≈0.8616
-
Percentage dissociation: α×100≈86.16%
