Question
Question: The reagent A is...
The reagent A is
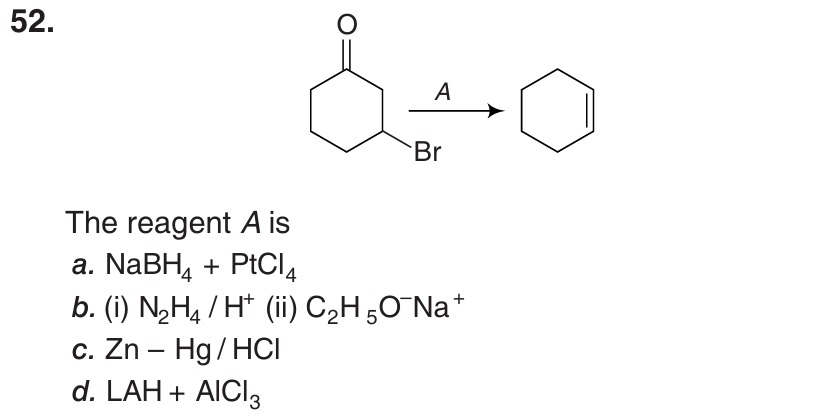
NaBH₄ + PtCl₄
(i) N₂H₄ / H⁺ (ii) C₂H₅O⁻Na⁺
Zn – Hg / HCl
LAH + AlCl₃
(i) N₂H₄ / H⁺ (ii) C₂H₅O⁻Na⁺
Solution
The reaction transforms 2-bromocyclohexanone into cyclohexene. The starting material is an α-bromoketone. The product is a cyclic alkene. This transformation involves the removal of the carbonyl group and the bromine atom, followed by the formation of a double bond. Specifically, the oxygen of the carbonyl group is removed, the bromine atom is removed, and a hydrogen atom is removed from the carbon adjacent to the bromine atom, resulting in the formation of a double bond between the carbon that was part of the carbonyl group and the carbon that was bonded to the bromine.
Let's examine the given reagents:
a. NaBH₄ + PtCl₄: NaBH₄ reduces ketones to alcohols. PtCl₄ combined with NaBH₄ can be used for reduction, but it is not a standard reagent for this transformation.
b. (i) N₂H₄ / H⁺ (ii) C₂H₅O⁻Na⁺: This is a two-step reaction. The first step, reaction with N₂H₄ in acidic medium, forms the hydrazone of 2-bromocyclohexanone.
O N-NH₂
/ \ //
C \ C \
/ \ \ $\xrightarrow{N_2H_4/H^+}$ / \ \
CH₂ CH-Br CH₂ CH-Br
| | | |
CH₂---CH₂ CH₂---CH₂
The second step involves treating the hydrazone with a strong base, C₂H₅O⁻Na⁺. This is the condition for Wolff-Kishner reduction, which typically reduces a carbonyl group to a methylene group. However, in the presence of an alpha-leaving group like bromine, the reaction pathway is altered. The strong base abstracts a proton from the NH₂ group, and the resulting anion undergoes tautomerization to form a carbanion at the carbon that was originally the carbonyl carbon.
N-NH₂ N-NH⁻ N=NH
// $\xrightarrow{B^-}$ // $\rightleftharpoons$ /
C \ C \ C⁻ \
/ \ \ / \ \ / \ \
CH₂ CH-Br CH₂ CH-Br CH₂ CH-Br
| | | | | |
CH₂---CH₂ CH₂---CH₂ CH₂---CH₂
The carbanion is at the carbon adjacent to the carbon bearing the bromine atom. This carbanion can undergo intramolecular elimination of the bromine atom, forming a double bond and eliminating nitrogen gas. The carbanion attacks the hydrogen on the adjacent carbon (which is bonded to Br), leading to the elimination of HBr and N₂. However, a more plausible mechanism involves the carbanion eliminating N₂ to form a carbene, which is then reduced, or a direct elimination of N₂ and Br⁻ to form a double bond.
A known reaction is the Shapiro reaction, which involves the reaction of tosylhydrazones with strong bases to form vinyl anions, which then undergo elimination of nitrogen. However, this is not a tosylhydrazone.
Considering the transformation from 2-bromocyclohexanone to cyclohexene, the reaction involves the removal of the carbonyl group, the bromine, and a hydrogen atom to form a double bond. The Wolff-Kishner reduction conditions with an alpha-bromo ketone are known to result in the formation of an alkene. The strong base in the second step promotes the elimination of the nitrogen from the hydrazone and the bromine atom, forming the double bond between the original carbonyl carbon and the carbon bearing the bromine.
c. Zn – Hg / HCl: This is the Clemmensen reduction, which reduces ketones and aldehydes to alkanes under acidic conditions. It is not suitable for this transformation as it would reduce the carbonyl group to a CH₂ group and the bromine would likely be removed as HBr under acidic conditions, possibly leading to cyclohexane or cyclohexene depending on the position of the double bond formation. However, the product is specifically cyclohexene.
d. LAH + AlCl₃: LAH (Lithium aluminium hydride) is a strong reducing agent that reduces ketones to alcohols. AlCl₃ is a Lewis acid that can activate the carbonyl group towards reduction. However, this combination is typically used for the reduction of esters, carboxylic acids, and amides to alcohols or amines. It would reduce the ketone to an alcohol, and it might also affect the carbon-bromine bond.
Revisiting option b, the reaction of α-haloketones with hydrazine and base (modified Wolff-Kishner conditions) can lead to the formation of alkenes. The mechanism involves the formation of the hydrazone, followed by treatment with a strong base. The base abstracts a proton from the NH₂ group, and the resulting anion undergoes tautomerization. The carbanion formed at the original carbonyl carbon is adjacent to the carbon bearing the bromine. Elimination of nitrogen and bromine occurs to form the alkene.
The reaction is a form of elimination and reduction. The carbonyl group is effectively removed, and the bromine atom is also removed, with the formation of a double bond. The conditions in option b are consistent with a reaction that leads to the formation of cyclohexene from 2-bromocyclohexanone.
