Question
Question: 52. Binding energy per nucleon vs mass number cure for nuclei is shown in the figure. W, X, Y and Z ...
- Binding energy per nucleon vs mass number cure for nuclei is shown in the figure. W, X, Y and Z are four nuclei indicated on the curve. The process that would release energy is:
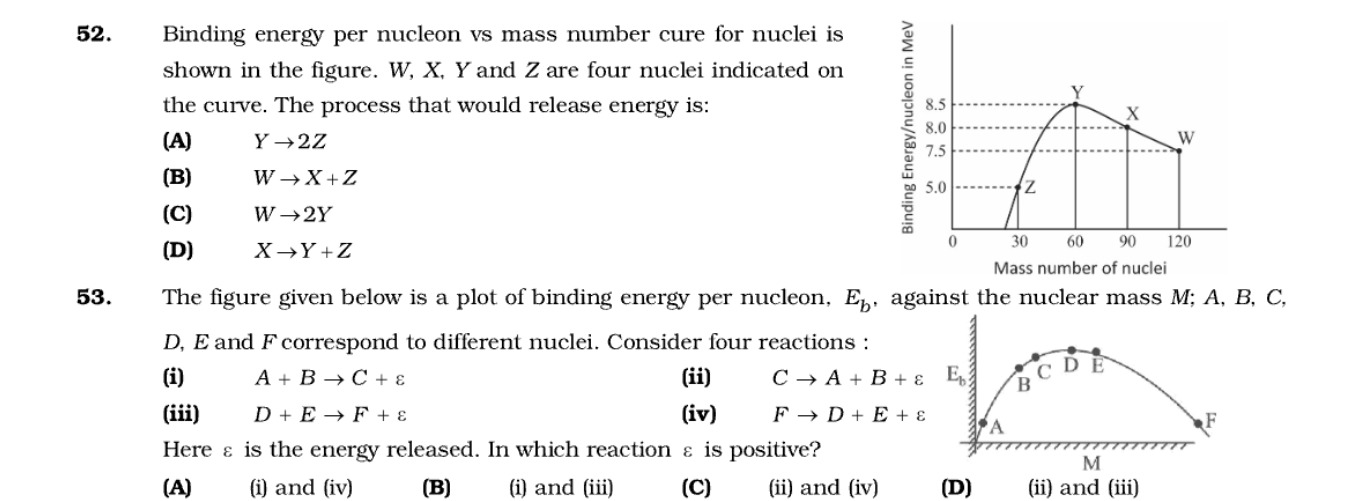
Y → 2Z
W → X+Z
W → 2Y
X → Y+Z
C
Solution
Energy is released in a nuclear reaction if the total binding energy of the products is greater than the total binding energy of the reactants. The total binding energy of a nucleus is given by the product of its mass number (A) and the binding energy per nucleon (Eb).
From the graph for question 52:
- Nucleus Y: Mass number ≈60, Binding energy per nucleon (EbY) ≈8.5 MeV.
- Nucleus X: Mass number ≈90, Binding energy per nucleon (EbX) ≈8.0 MeV.
- Nucleus W: Mass number ≈120, Binding energy per nucleon (EbW) ≈7.5 MeV.
- Nucleus Z: Mass number ≈30, Binding energy per nucleon (EbZ) ≈5.0 MeV.
Let's analyze each option: (A) Y→2Z: Mass number conservation: 60→2×30=60. Total binding energy of Y = 60×EbY≈60×8.5=510 MeV. Total binding energy of 2Z = 2×(30×EbZ)≈2×(30×5.0)=300 MeV. Energy released = B.E.products−B.E.reactants=300−510=−210 MeV. (Energy absorbed)
(B) W→X+Z: Mass number conservation: 120→90+30=120. Total binding energy of W = 120×EbW≈120×7.5=900 MeV. Total binding energy of X+Z = (90×EbX)+(30×EbZ)≈(90×8.0)+(30×5.0)=720+150=870 MeV. Energy released = 870−900=−30 MeV. (Energy absorbed)
(C) W→2Y: Mass number conservation: 120→2×60=120. Total binding energy of W = 120×EbW≈120×7.5=900 MeV. Total binding energy of 2Y = 2×(60×EbY)≈2×(60×8.5)=2×510=1020 MeV. Energy released = 1020−900=120 MeV. (Energy released)
(D) X→Y+Z: Mass number conservation: 90→60+30=90. Total binding energy of X = 90×EbX≈90×8.0=720 MeV. Total binding energy of Y+Z = (60×EbY)+(30×EbZ)≈(60×8.5)+(30×5.0)=510+150=660 MeV. Energy released = 660−720=−60 MeV. (Energy absorbed)
The process that releases energy is W→2Y. This is a fission process where a heavier nucleus (W) splits into lighter nuclei (Y), and the products (Y) are more tightly bound (higher binding energy per nucleon) than the reactant (W).
