Question
Question: The end product of the reaction is: + NBS $\longrightarrow$ X $\xrightarrow{Mg/ether}$ Y $\xrightar...
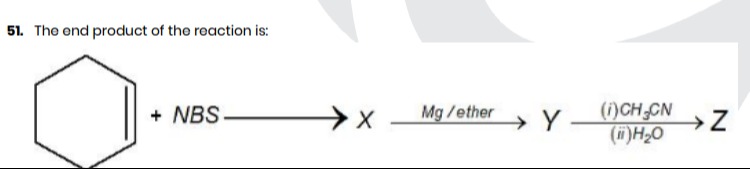
The end product of the reaction is:
- NBS ⟶ X Mg/ether Y (i)CH3CN(ii)H2O Z
Answer
The end product Z is 1-(cyclohex-2-en-1-yl)ethan-1-one.
Explanation
Solution
The reaction proceeds through three main steps:
- Allylic bromination of cyclohexene with NBS to form 3-bromocyclohexene (X).
- Formation of a Grignard reagent from 3-bromocyclohexene by reaction with Mg in ether, yielding cyclohex-2-en-1-ylmagnesium bromide (Y).
- Reaction of the Grignard reagent Y with acetonitrile (CH₃CN) followed by hydrolysis, which is a standard method for synthesizing ketones from nitriles and Grignard reagents. This yields 1-(cyclohex-2-en-1-yl)ethan-1-one (Z).
