Question
Question: Which of the following will liberate a colourless gas when treated with sodium metal?...
Which of the following will liberate a colourless gas when treated with sodium metal?
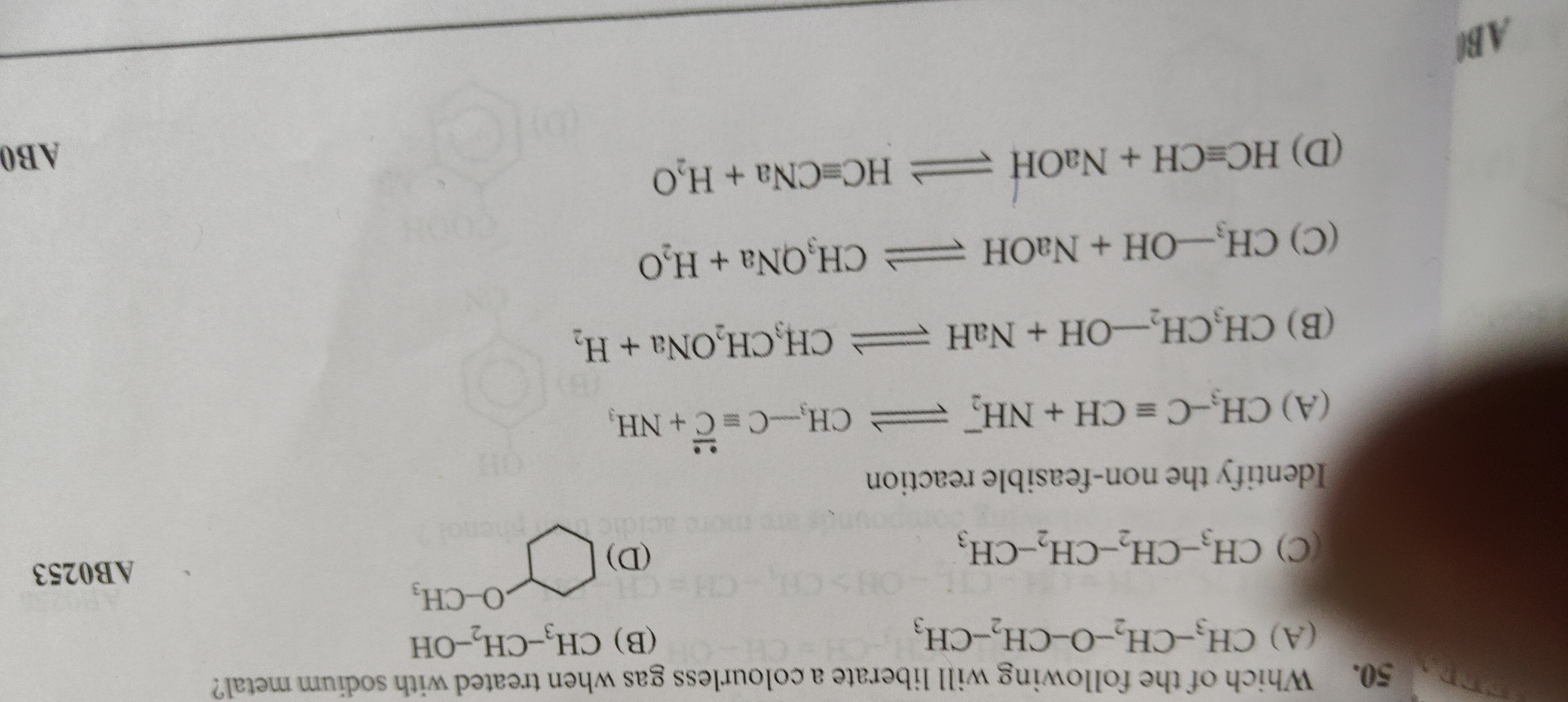
A
CH₃-CH₂-O-CH₂-CH₃
B
CH₃-CH₂-OH
C
CH₃-CH₂-CH₂-CH₃
D
Cyclohexanol
Answer
B, D
Explanation
Solution
Sodium metal reacts with compounds containing acidic hydrogen atoms to liberate hydrogen gas (H₂), which is a colorless gas.
- (A) Diethyl ether (CH₃-CH₂-O-CH₂-CH₃): Ethers do not have acidic hydrogens.
- (B) Ethanol (CH₃-CH₂-OH): Alcohols have an acidic hydrogen atom in the hydroxyl (-OH) group. This hydrogen can be displaced by sodium metal, producing hydrogen gas. 2CH3CH2OH+2Na→2CH3CH2ONa+H2↑
- (C) Butane (CH₃-CH₂-CH₂-CH₃): Alkanes do not have acidic hydrogens.
- (D) Cyclohexanol: Like other alcohols, cyclohexanol has an acidic hydrogen atom in the hydroxyl (-OH) group, which reacts with sodium metal to liberate hydrogen gas. 2C6H11OH+2Na→2C6H11ONa+H2↑
Both (B) and (D) will liberate a colorless gas (H₂) when treated with sodium metal.
