Question
Question: Number of compounds in the following which will give red colour when subjected to Victor Mayer test ...
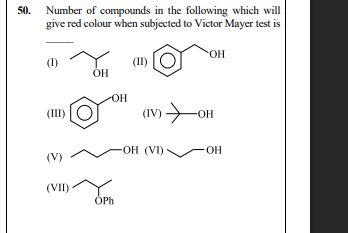
Number of compounds in the following which will give red colour when subjected to Victor Mayer test is
(I) OH
(II) OH
(III) OH
(IV) OH
(V) -OH (VI) -OH
(VII) OPh
3
Solution
The Victor Meyer test is a chemical test used to distinguish between primary, secondary, and tertiary alcohols. The test involves three main steps:
-
Formation of Alkyl Iodide: The alcohol is reacted with red phosphorus and iodine (or HI) to form the corresponding alkyl iodide.
R-OH + PI₃ → R-I + H₃PO₃(orR-OH + HI → R-I + H₂O) -
Formation of Nitroalkane: The alkyl iodide is then reacted with silver nitrite (AgNO₂) to form a nitroalkane.
R-I + AgNO₂ → R-NO₂ + AgI -
Reaction with Nitrous Acid (HNO₂): The nitroalkane is then treated with nitrous acid (a freshly prepared mixture of NaNO₂ and HCl) and finally made alkaline with KOH or NaOH. The color developed depends on the type of alcohol:
-
Primary (1°) Alcohol: Forms a primary nitroalkane (RCH₂NO₂). This reacts with HNO₂ to form a nitrolic acid (R-C(=NOH)-NO₂), which is acidic and dissolves in alkali (NaOH/KOH) to produce a red color.
R-CH₂-NO₂ + HONO → R-C(=NOH)-NO₂(Nitrolic acid) + H₂OR-C(=NOH)-NO₂ + NaOH → Red colored salt -
Secondary (2°) Alcohol: Forms a secondary nitroalkane (R₂CHNO₂). This reacts with HNO₂ to form a pseudonitrole (R₂C(NO)-NO₂), which is a blue-colored compound. It does not dissolve in alkali to give a different color.
R₂CH-NO₂ + HONO → R₂C(NO)-NO₂(Pseudonitrole) + H₂O -
Tertiary (3°) Alcohol: Forms a tertiary nitroalkane (R₃CNO₂). Since there are no α-hydrogens, it does not react with HNO₂. Thus, the solution remains colorless.
R₃C-NO₂ + HONO → No reaction
-
Now, let's classify each given compound and predict its Victor Meyer test result:
(I) CH₃-CH(OH)-CH₃ (Propan-2-ol): This is a secondary alcohol. It will give a blue color.
(II) C₆H₅-CH₂-OH (Benzyl alcohol): This is a primary alcohol. It will give a red color.
(III) C₆H₅-OH (Phenol): This is a phenol, not an alcohol. The Victor Meyer test is not applicable to phenols. It will not give a characteristic red color.
(IV) (CH₃)₃C-OH (2-Methylpropan-2-ol or tert-butyl alcohol): This is a tertiary alcohol. It will be colorless.
(V) CH₃-CH₂-CH₂-OH (Propan-1-ol): This is a primary alcohol. It will give a red color.
(VI) CH₃-CH₂-OH (Ethanol): This is a primary alcohol. It will give a red color.
(VII) CH₃-CH(OPh)-CH₂-CH₃ (2-Phenoxybutane): This is an ether, not an alcohol. The Victor Meyer test is not applicable to ethers. It will not give a characteristic red color.
The compounds that will give a red color are (II), (V), and (VI). Therefore, the number of compounds that will give a red color when subjected to the Victor Meyer test is 3.
Explanation of the solution: The Victor Meyer test distinguishes primary, secondary, and tertiary alcohols. Primary alcohols yield red color, secondary alcohols yield blue color, and tertiary alcohols remain colorless. Phenols and ethers do not respond to this test.
- (I) Propan-2-ol: Secondary alcohol, gives blue color.
- (II) Benzyl alcohol: Primary alcohol, gives red color.
- (III) Phenol: Not an alcohol, no specific color.
- (IV) 2-Methylpropan-2-ol: Tertiary alcohol, colorless.
- (V) Propan-1-ol: Primary alcohol, gives red color.
- (VI) Ethanol: Primary alcohol, gives red color.
- (VII) 2-Phenoxybutane: Ether, no specific color.
Compounds giving red color are (II), (V), and (VI). Total count is 3.
