Question
Question: The correct order of decreasing basic strengths of x, y and z is:...
The correct order of decreasing basic strengths of x, y and z is:
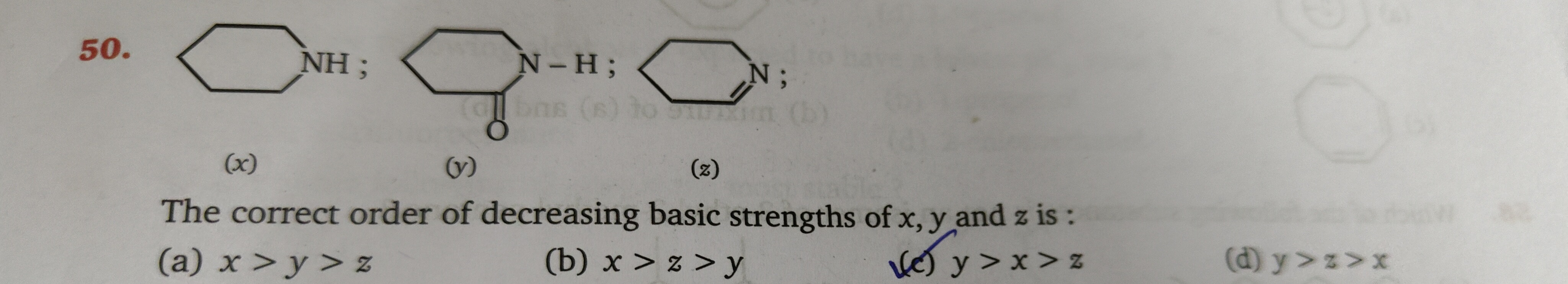
A
x > y > z
B
x > z > y
C
y > x > z
D
y > z > x
Answer
x > z > y
Explanation
Solution
The basicity of a nitrogen compound is determined by the availability of the lone pair of electrons on the nitrogen atom.
- Compound (x) is cyclohexylamine, a secondary aliphatic amine. The nitrogen atom is sp3 hybridized, and its lone pair is readily available for protonation. The cyclohexyl ring is electron-donating, enhancing the electron density on nitrogen.
- Compound (y) is a cyclic amide (lactam). The nitrogen atom is sp2 hybridized and its lone pair is delocalized through resonance with the adjacent carbonyl group (C=O). This delocalization significantly reduces the availability of the lone pair for protonation, making amides very weak bases.
- Compound (z) is a cyclic imine. The nitrogen atom is sp2 hybridized. The lone pair resides in an sp2 orbital, which is less available than in an sp3 orbital. However, unlike amides, the lone pair is not delocalized by resonance with a carbonyl group. Thus, imines are less basic than amines but more basic than amides.
Therefore, the order of decreasing basic strengths is: Amine (x) > Imine (z) > Amide (y) This translates to the order x > z > y.
