Question
Question: Calculate the value of $\Delta G$ for the following reaction at 300 K, $HgS_{(s)} + O_{2(g)} \right...
Calculate the value of ΔG for the following reaction at 300 K,
HgS(s)+O2(g)→Hg(l)+SO2(g) if ΔH=−240 kJ • and ΔS=36.5 J K−1.
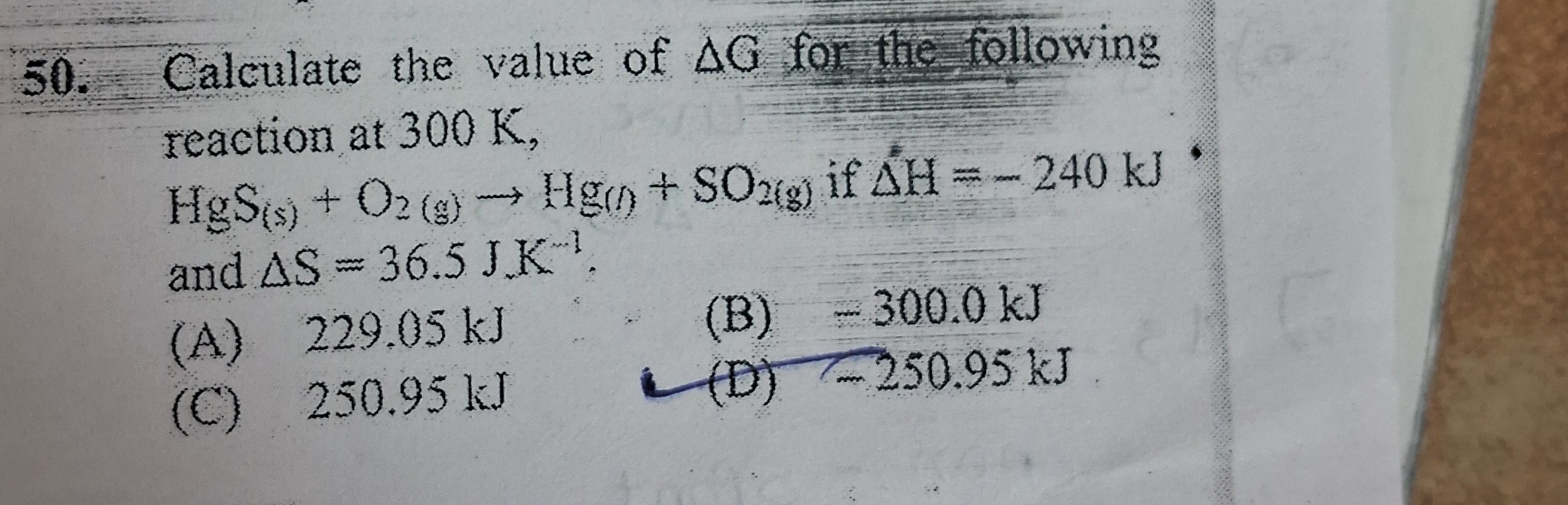
A
229.05 kJ
B
- 300.0 kJ
C
250.95 kJ
D
-250.95 kJ
Answer
-250.95 kJ
Explanation
Solution
The free energy change is given by:
ΔG=ΔH−TΔSGiven:
- ΔH=−240kJ
- ΔS=36.5J K−1=0.0365kJ K−1
- T=300K
Substitute the values:
ΔG=−240kJ−300×0.0365kJ ΔG=−240kJ−10.95kJ=−250.95kJCore Explanation:
Convert ΔS to kJ/K, substitute in ΔG=ΔH−TΔS and calculate.
