Question
Question: Which among the following compounds contains amino group?...
Which among the following compounds contains amino group?
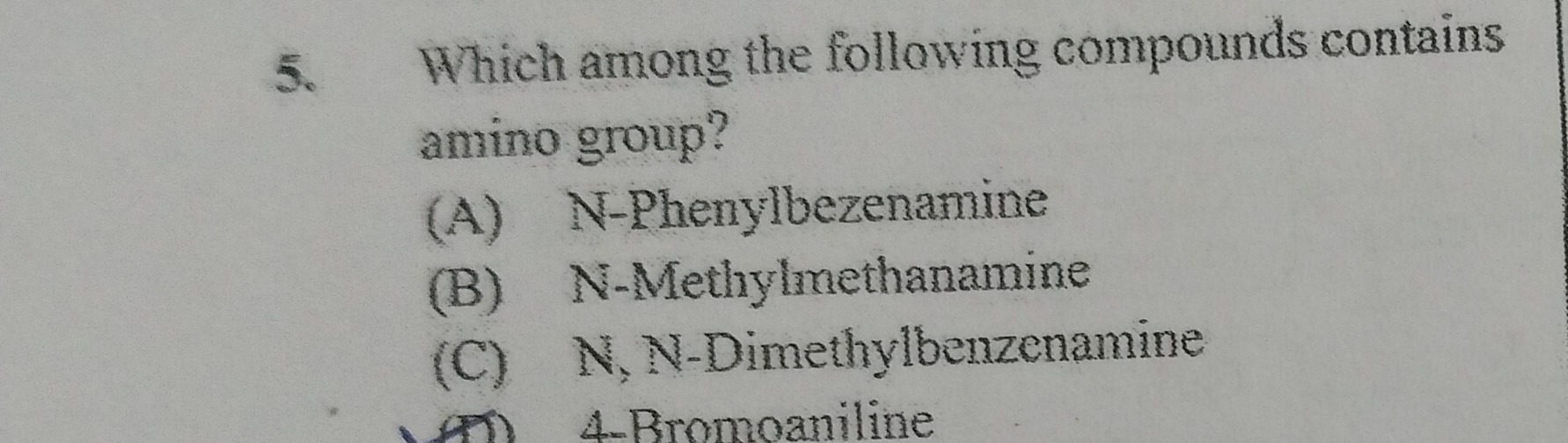
A
N-Phenylbezenamine
B
N-Methylmethanamine
C
N,N-Dimethylbenzenamine
D
4-Bromoaniline
Answer
4-Bromoaniline
Explanation
Solution
- Option A: N-Phenylbenzenamine (diphenylamine) has the structure Ph–NH–Ph. The NH is bonded to two phenyl groups and is not in the form of a free –NH₂ group.
- Option B: N-Methylmethanamine is essentially CH₃NHCH₃, a secondary amine, and does not have the free –NH₂ moiety.
- Option C: N,N-Dimethylbenzenamine (or N,N-Dimethylaniline) is a tertiary amine, lacking any hydrogen directly attached to nitrogen.
- 4-Bromoaniline: This compound has the structure 4-Br–C₆H₄–NH₂, where the –NH₂ group is clearly present.
Thus, the only compound that contains an amino (–NH₂) group is 4-Bromoaniline.
