Question
Question: When $M_1$ gram of ice at -5° C (specific heat = 0.5 cal g⁻¹ °C⁻¹) is added to $M_2$ gram of water a...
When M1 gram of ice at -5° C (specific heat = 0.5 cal g⁻¹ °C⁻¹) is added to M2 gram of water at 50°C (specific heat = 1 cal g⁻¹ °C⁻¹), finally no ice is left and the water is at 0°C. The value of latent heat of ice, in cal g⁻¹ is:
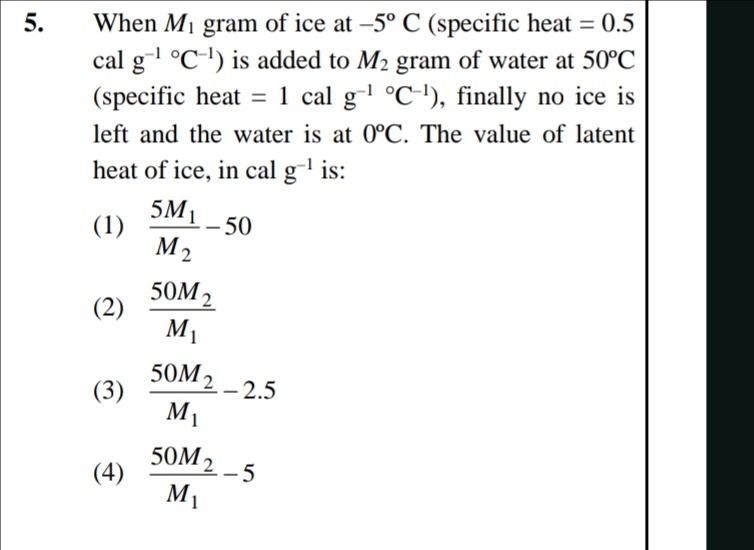
M25M1−50
M150M2
M150M2−2.5
M150M2−5
(3) M150M2−2.5
Solution
The problem involves the principle of calorimetry, which states that in an isolated system, the total heat lost by hotter bodies is equal to the total heat gained by colder bodies. In this case, the water at 50∘C loses heat, and the ice at −5∘C gains heat. The final state is water at 0∘C, meaning all ice has melted.
- Heat gained by ice (Qgain):
The ice undergoes two processes to reach the final state:
- Heating of ice: Ice at −5∘C heats up to 0∘C.
Heat gained, Q1=M1×sice×ΔTice
Q1=M1×0.5 cal g−1∘C−1×(0−(−5))∘C
Q1=M1×0.5×5=2.5M1 cal
- Melting of ice: Ice at 0∘C melts into water at 0∘C.
Heat gained, Q2=M1×Lf
where Lf is the latent heat of fusion of ice.
Total heat gained by ice, Qgain=Q1+Q2=2.5M1+M1Lf.
- Heat lost by water (Qlost):
The water at 50∘C cools down to 0∘C.
Heat lost, Qlost=M2×swater×ΔTwater
Qlost=M2×1 cal g−1∘C−1×(50−0)∘C
Qlost=M2×1×50=50M2 cal
- Applying the principle of calorimetry:
Heat Lost = Heat Gained
Qlost=Qgain
50M2=2.5M1+M1Lf
- Solving for Lf:
Rearrange the equation to find Lf:
M1Lf=50M2−2.5M1
Lf=M150M2−2.5M1
Lf=M150M2−M12.5M1
Lf=M150M2−2.5 cal g−1
