Question
Question: The $\widehat{CNC}$ bond angle in $CH_3NCS$ is:...
The CNC bond angle in CH3NCS is:
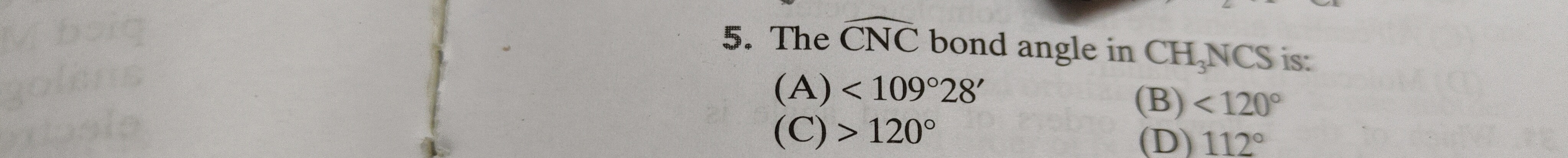
A
< 109∘28′
B
<120∘
C
120∘
D
112∘
Answer
(C)
Explanation
Solution
The nitrogen atom in CH3NCS exhibits resonance. While the most common Lewis structure suggests sp2 hybridization with a lone pair leading to an angle less than 120∘, a significant resonance contributor involves a triple bond to nitrogen, implying sp hybridization and a linear angle of 180∘. Experimental data shows the actual CNC bond angle to be approximately 141.6∘, which is greater than 120∘, indicating a dominant influence of the sp hybridized linear character.
