Question
Question: The relative yield of the following alkenyl bromides from the reaction of 1,3-butadiene with HBr (da...
The relative yield of the following alkenyl bromides from the reaction of 1,3-butadiene with HBr (dark, N₂ atmosphere) at -15°C is
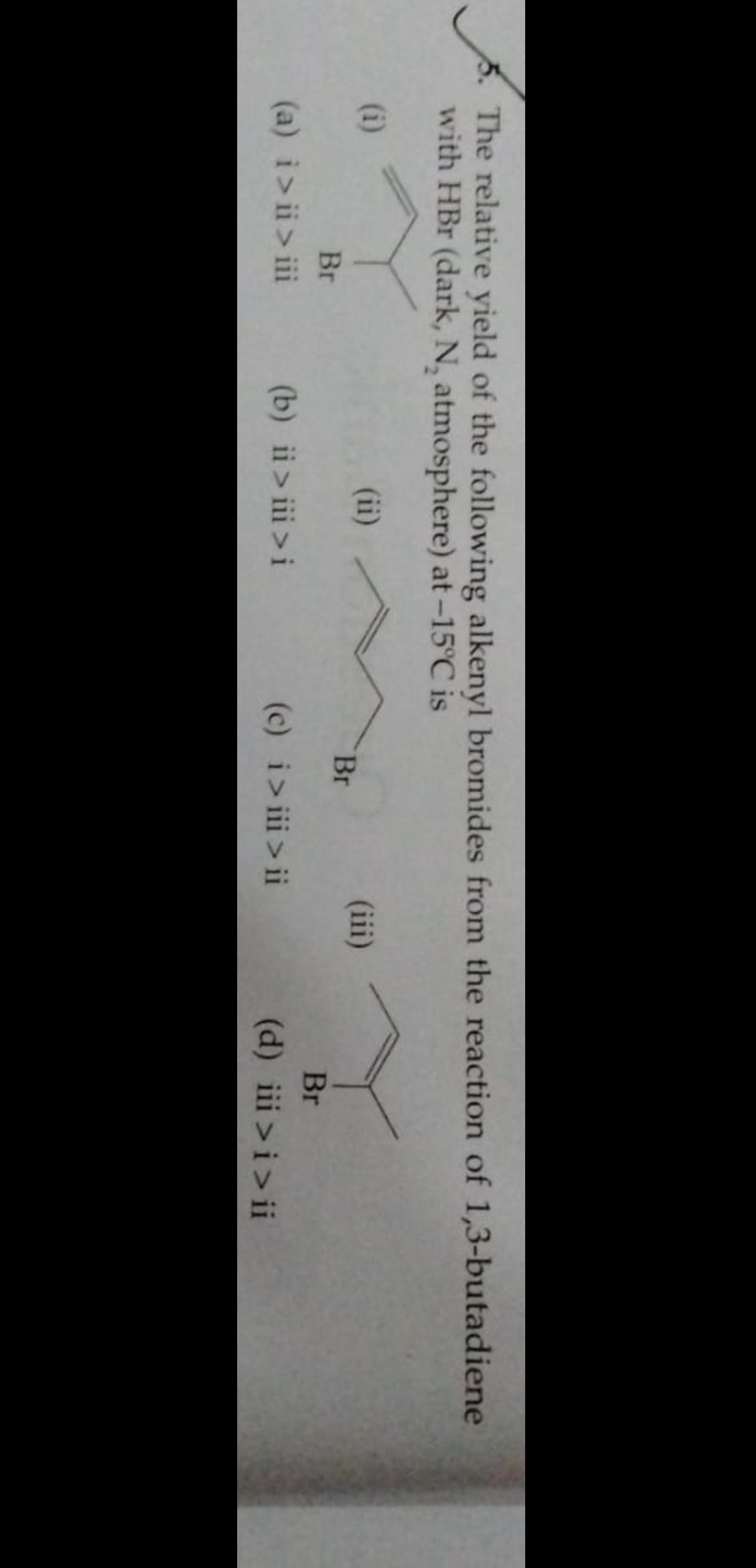
i>ii > iii
ii>iii>i
i > iii > ii
iii >i>ii
i>ii > iii
Solution
The reaction of 1,3-butadiene with HBr proceeds via electrophilic addition. The first step is the protonation of the diene to form a resonance-stabilized allylic carbocation:
CH2=CH−CH=CH2+H+→[CH3−CH+−CH=CH2↔CH3−CH=CH−CH2+]
The allylic carbocation is a resonance hybrid with the positive charge distributed on C2 and C4. The resonance structure with the positive charge on the more substituted carbon (secondary carbon C2) is more stable, contributing more to the hybrid.
The second step is the nucleophilic attack of Br− on the carbocation.
Attack at C2 (1,2-addition): CH3−CH+−CH=CH2+Br−→CH3−CH(Br)−CH=CH2 (Product i)
Attack at C4 (1,4-addition): CH3−CH=CH−CH2++Br−→CH3−CH=CH−CH2Br (Product ii)
At low temperature (-15°C), the reaction is under kinetic control. The kinetic product is the one formed fastest. The rate of formation is determined by the activation energy of the transition state. The transition state leading to the 1,2-addition product involves the attack of Br− at C2, which has a higher partial positive charge due to the greater contribution of the resonance structure with the positive charge on the secondary carbon. This leads to a lower activation energy for the 1,2-addition. Therefore, the 1,2-addition product (i) is the major kinetic product.
Product (iii) is 2-bromo-2-butene: CH3−C(Br)=CH−CH3. This product is not formed by direct 1,2 or 1,4 addition of HBr to 1,3-butadiene. It is a vinylic bromide. Its formation is unlikely under these conditions. However, if we assume it is formed, its stability is determined by the substitution of the double bond. Product (iii) has a trisubstituted double bond. Product (ii) has a disubstituted double bond. Product (i) has a monosubstituted double bond. The stability order of the double bond is trisubstituted > disubstituted > monosubstituted. So, the thermodynamic stability order is (iii) > (ii) > (i).
At -15°C, the reaction is under kinetic control, so the kinetic product (i) is favored. While some amount of thermodynamic product may also be formed, the kinetic product is the major product. So, the yield of (i) should be the highest.
The final answer is i>ii > iii.
