Question
Question: Salt A + S $\xrightarrow{BaCl_2}$ B White ppt. A is paramagnetic in nature and contains about 55% K...
Salt A + S BaCl2 B White ppt.
A is paramagnetic in nature and contains about 55% K. Thus, A is :
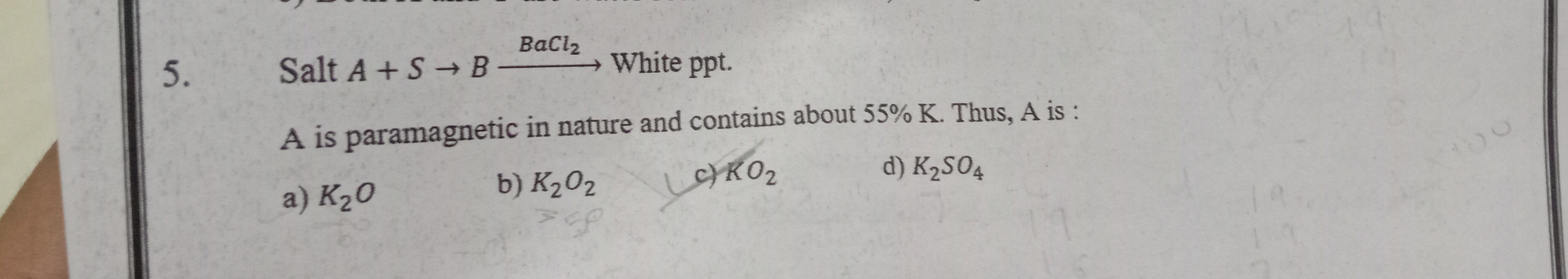
A
K₂O
B
K₂O₂
C
KO₂
D
K₂SO₄
Answer
KO₂
Explanation
Solution
The compound A is identified based on its properties: paramagnetism and percentage of potassium.
-
Percentage of Potassium: The molar mass of K is approximately 39.1 g/mol, and O is 16.0 g/mol.
- K₂O: Molar mass ≈ 94.2 g/mol. %K ≈ (2 × 39.1 / 94.2) × 100 ≈ 83.0%.
- K₂O₂: Molar mass ≈ 110.2 g/mol. %K ≈ (2 × 39.1 / 110.2) × 100 ≈ 71.0%.
- KO₂: Molar mass ≈ 71.1 g/mol. %K ≈ (39.1 / 71.1) × 100 ≈ 55.0%. Option (c) KO₂ matches the given condition of containing about 55% K.
-
Paramagnetic Nature:
- K₂O contains K⁺ and O²⁻ ions, both diamagnetic.
- K₂O₂ contains K⁺ and O₂²⁻ (peroxide ion). The O₂²⁻ ion has all electrons paired, making it diamagnetic.
- KO₂ contains K⁺ and O₂⁻ (superoxide ion). The superoxide ion (O₂⁻) has 13 valence electrons. According to molecular orbital theory, it has one unpaired electron in the π∗ antibonding orbital, making KO₂ paramagnetic.
- K₂SO₄ contains K⁺ and SO₄²⁻ ions, both diamagnetic. Option (c) KO₂ is paramagnetic.
Both conditions are met by KO₂. The reaction with sulfur, followed by addition of BaCl₂, producing a white precipitate, is consistent with KO₂ forming potassium sulfite (K₂SO₃) or potassium thiosulfate (K₂S₂O₃), which then precipitate as BaSO₃ or BaS₂O₃, respectively, upon addition of BaCl₂.
