Question
Question: One mole of an ideal monoatomic gas is caused to go through the cycle shown in figure. Then the chan...
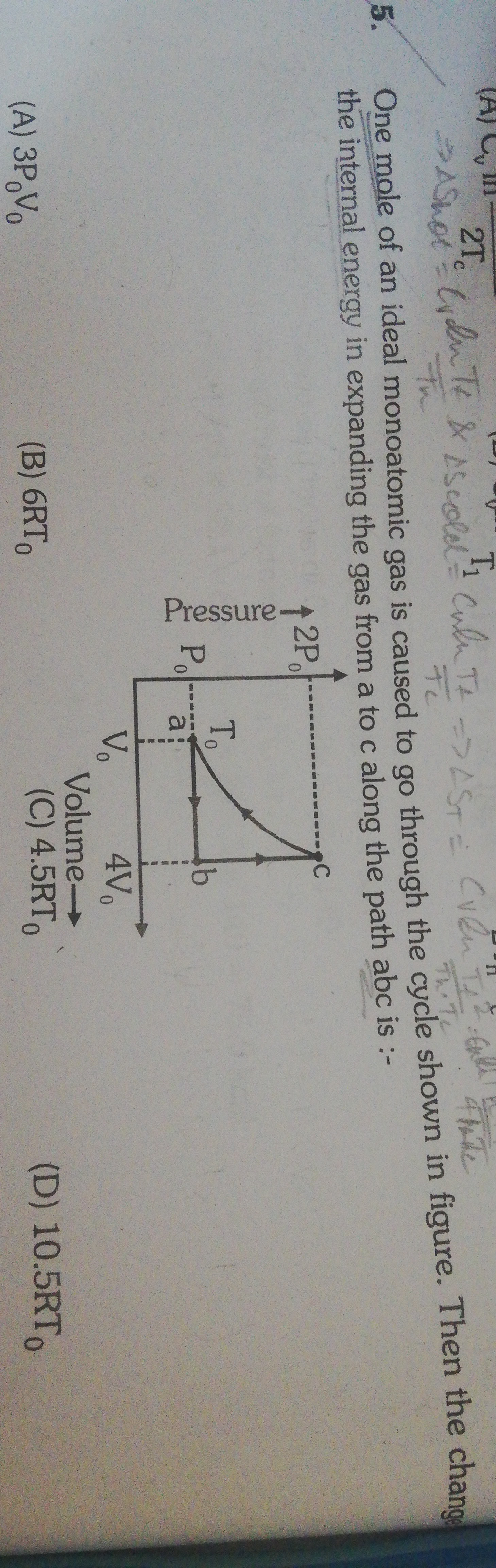
One mole of an ideal monoatomic gas is caused to go through the cycle shown in figure. Then the change the internal energy in expanding the gas from a to c along the path abc is :-
3P₀V₀
6RT₀
4.5RT₀
10.5RT₀
10.5RT₀
Solution
The internal energy of an ideal gas depends only on its temperature. For one mole of a monoatomic ideal gas, the internal energy is given by U=23RT. The change in internal energy is ΔU=23R(Tf−Ti).
From the P-V diagram, state 'a' has pressure P0 and volume V0. Assuming n=1 mole, P0V0=RTa. Let Ta=T0. So, P0V0=RT0.
State 'c' has pressure 2P0 and volume 4V0. The temperature at state 'c' is Tc=RPcVc=R(2P0)(4V0)=R8P0V0. Substituting P0V0=RT0, we get Tc=R8RT0=8T0.
The change in internal energy from 'a' to 'c' is ΔU=23R(Tc−Ta)=23R(8T0−T0)=23R(7T0)=10.5RT0. The path taken ('abc') does not affect the change in internal energy, as it is a state function.
