Question
Question: If $d_{xy}$ orbital of atom (A) and $p_y$ orbital of atom (B) form pi ($\pi$) bond along a molecular...
If dxy orbital of atom (A) and py orbital of atom (B) form pi (π) bond along a molecular axis, then which condition(s) is/are NOT suitable along same molecular axis by combination of pz + pz orbital of (A) and (B) atoms respectively:
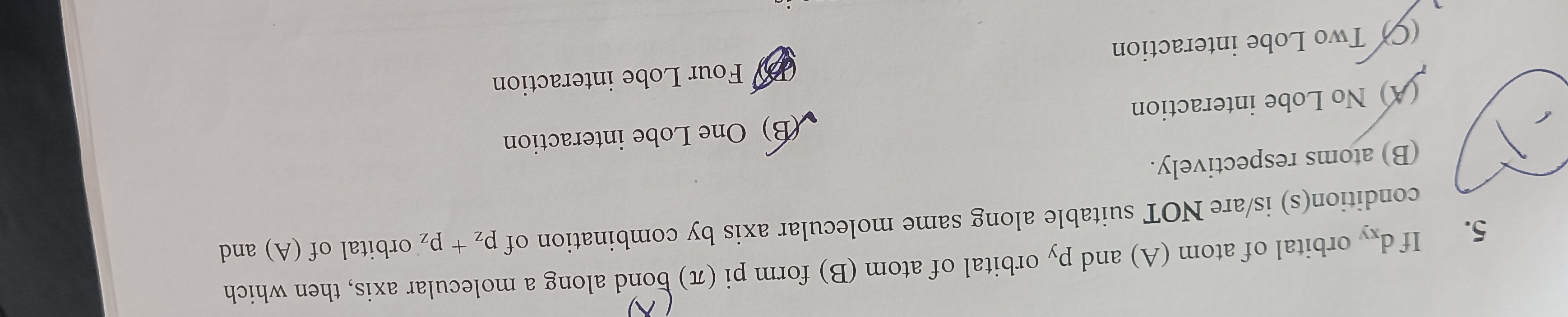
No Lobe interaction
Two Lobe interaction
One Lobe interaction
Four Lobe interaction
A, B, D
Solution
To solve this problem, we first need to determine the molecular axis based on the formation of a π bond between the dxy orbital of atom (A) and the py orbital of atom (B). Then, we will analyze the interaction of pz and pz orbitals along this determined molecular axis.
Step 1: Determine the molecular axis for dxy and py forming a π bond.
For a π bond to form, the internuclear axis must lie in a nodal plane of the overlapping orbitals, and the orbitals must have the same symmetry with respect to this axis.
-
Consider x-axis as the molecular axis:
- For py orbital: Its lobes are along the y-axis. The xz-plane is a nodal plane for py, and the x-axis lies within this plane. Thus, py has π symmetry with respect to the x-axis.
- For dxy orbital: Its lobes are in the xy-plane, between the x and y axes. The xz-plane is a nodal plane for dxy, and the x-axis lies within this plane. Thus, dxy has π symmetry with respect to the x-axis.
- Since both py and dxy have π symmetry with respect to the x-axis and share the same nodal plane (xz-plane), they can form a π bond along the x-axis. This is a suitable condition.
-
Consider y-axis as the molecular axis:
- For py orbital: Its lobes are along the y-axis. If the y-axis is the internuclear axis, the overlap would be head-on, forming a σ bond, not a π bond.
- Therefore, the y-axis is not a suitable molecular axis for π bond formation between dxy and py.
-
Consider z-axis as the molecular axis:
- For py orbital: Its lobes are along the y-axis. The xz-plane is a nodal plane for py, and the z-axis lies within this plane. Thus, py has π symmetry with respect to the z-axis.
- For dxy orbital: Its lobes are in the xy-plane. Both the xz-plane and yz-plane are nodal planes for dxy, and the z-axis lies within both. This indicates δ symmetry with respect to the z-axis.
- Since py has π symmetry and dxy has δ symmetry with respect to the z-axis, they cannot form a bond.
- Therefore, the z-axis is not a suitable molecular axis for π bond formation between dxy and py.
From this analysis, the molecular axis along which dxy and py form a π bond must be the x-axis.
Step 2: Analyze the combination of pz+pz orbitals along the x-axis.
- The pz orbital has lobes along the z-axis. Its nodal plane is the xy-plane.
- When two pz orbitals approach each other along the x-axis (the internuclear axis), their lobes are oriented perpendicular to the internuclear axis.
- The positive lobe of one pz orbital can overlap laterally with the positive lobe of the other pz orbital (e.g., above the xy-plane), and similarly, their negative lobes can overlap laterally (e.g., below the xy-plane).
- This type of lateral overlap forms a π bond. The electron density is concentrated in two regions, one above and one below the internuclear axis (the x-axis). The xy-plane becomes the nodal plane containing the x-axis.
Step 3: Evaluate the given conditions for pz+pz overlap along the x-axis.
A π bond is characterized by "Two Lobe interaction" (meaning two regions of constructive overlap).
- (A) No Lobe interaction: This condition implies no bond formation. However, pz+pz along the x-axis forms a π bond, so there is lobe interaction. Therefore, this condition is NOT suitable.
- (B) One Lobe interaction: This condition typically describes a σ bond (head-on overlap, one region of electron density). A π bond involves two regions of overlap. Therefore, this condition is NOT suitable.
- (C) Two Lobe interaction: This condition correctly describes a π bond, which is formed by pz+pz along the x-axis. Therefore, this condition IS suitable.
- (D) Four Lobe interaction: This condition describes a δ bond. A π bond does not involve four lobe interactions. Therefore, this condition is NOT suitable.
The question asks for the condition(s) that are NOT suitable. Based on our analysis, conditions (A), (B), and (D) are not suitable.
