Question
Question: $4CH_4 + O_2 \xrightarrow{Cu \atop 573k/1100atm}$...
4CH4+O2573k/1100atmCu
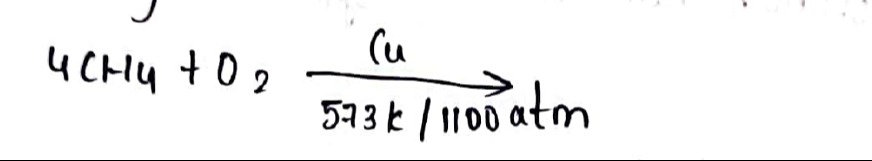
Methanol (CH3OH)
Solution
The given reaction is a controlled oxidation of methane.
Reaction Analysis:
The reaction involves methane (CH4) and oxygen (O2) under specific conditions:
- Catalyst: Copper (Cu)
- Temperature: 573 K
- Pressure: 1100 atm
These conditions are characteristic for the partial oxidation of methane to methanol. According to NCERT, methane is oxidized to methanol in the presence of copper at 573 K and 100 atm. The higher pressure (1100 atm) mentioned in the question is also a very high pressure, which favors the formation of methanol.
The balanced chemical equation for the formation of methanol from methane is:
2CH4(g)+O2(g)573K/100atmCu2CH3OH(l)
Stoichiometry Consideration:
The question provides the stoichiometry as 4CH4+O2. From the balanced equation, 1 mole of O2 reacts with 2 moles of CH4. In the given reaction, we have 1 mole of O2 and 4 moles of CH4. This means O2 is the limiting reactant. 1 mole of O2 will react with 2 moles of CH4 to produce 2 moles of CH3OH. The remaining 2 moles of CH4 will be unreacted. When asked for "the product" in such a reaction, it refers to the primary chemical species formed.
Product:
The primary product of this controlled oxidation of methane under these conditions is methanol.
Chemical Structure of Methanol:
Methanol (methyl alcohol) has the chemical formula CH3OH. SMILES: CO
Explanation:
Methane undergoes controlled oxidation in the presence of a copper catalyst at 573 K and high pressure (100-1100 atm) to yield methanol. The given stoichiometry indicates that oxygen is the limiting reactant, leading to the formation of methanol, with some unreacted methane remaining.
